
����ѡ������ȷ����
A. 1L0.5mol��L��1ϡ������1L1mol��L��1����������Һ��Ӧ�ų�57.3kJ��������
H2SO4(aq) +2NaOH(aq) = Na2SO4(aq) +2H2O(1)���� H = ��57.3 kJ�� mol-1
B. ij�����ʽ��NaHYˮ��Һ�Լ��ԣ���H2Y![]() 2H++Y2��
2H++Y2��
C. ������Һ�Լ��Ե�ԭ�������ӷ���ʽ�ɱ�ʾΪ��CO32��+2H2O![]() H2CO3+2OH��
H2CO3+2OH��
D���Զ��Ե缫���KCl��Һ�����ӷ���ʽΪ��2Cl��+2H2O ![]() H2��+2OH��+Cl2��
H2��+2OH��+Cl2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?���ϣ�þ��������й㷺��;����ش��й�þ���������⣺
��2011?���ϣ�þ��������й㷺��;����ش��й�þ���������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
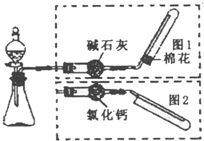 ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ���� ��ͼ��ʵ������ȡ�����װ�ã����з���װ����ͬ������ͼ���װ�������ף��ֱ���ͼ1��ͼ2��ʾ������ѡ������ȷ����
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
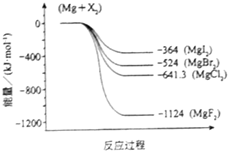 ����þ��һ�ֻ��õij������������Ź㷺����;����ͼ�ǽ���þ��±�ط�Ӧ�������仯ͼ����Ӧ��Ͳ����Ϊ298Kʱ���ȶ�״̬����
����þ��һ�ֻ��õij������������Ź㷺����;����ͼ�ǽ���þ��±�ط�Ӧ�������仯ͼ����Ӧ��Ͳ����Ϊ298Kʱ���ȶ�״̬�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2H2��g��+O2��g��=2H2O��l����H=-285.8kJ?mol-1 | ||
| B��CH4��g��+2O2��g��=CO2��g��+2H2O��l����H=+890.3kJ?mol-1 | ||
C��H2��g��+
| ||
| D��C��s��+2H2��g��=CH4��g����H=-74.8kJ?mol-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com