
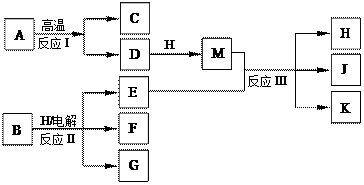
 E��G��Ӧ�����ӷ���ʽ�� ��
E��G��Ӧ�����ӷ���ʽ�� ��
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��
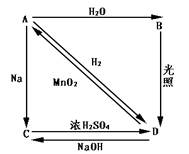
�� A �� B ��
A �� B ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ˮ����KI��Һ�У���Һ���ᷢ���仯 | B�����Ƶ���ˮ�ʻ���ɫ |
| C����ˮ�е���AgNO3��Һ���а�ɫ�������� | D�����õ���ˮ��ɫ��dz |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��
ʵ������������ʵ��װ��̽��Cl2���ʲ�ģ���Ʊ�Ưˮ��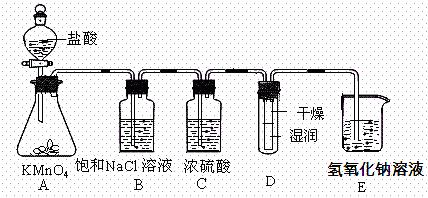
 __��
__�� ���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________
���µġ����жϸý����Ƿ��������������������ʵ�������ԭ��_________________�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ClO2�ķ��ӽṹ��V�ͣ����Ǽ��Է��� |
| B��ClO2����Ԫ���ԣ�4�ۣ����к�ǿ�������ԣ�������Ч��(�Ե�λ����õ��ӵ���Ŀ��ʾ)��Cl2��5�� |
| C��ClO2��Cl2��ϡ��Һ���ڻ������������й���Ч���ص㣬���������κ�Σ�� |
| D������Na2SO3��H2SO4�����������£���ԭNaClO3����ClO2����һ��Ӧ�Ļ�ѧ����ʽ�ɱ�ʾΪNa2SO3��2NaClO3��H2SO4====2Na2SO4��2ClO2����H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������������ˮ�ܽ⣬�������ữ���ټ���AgNO3��Һ�۲����������� |
| B������NaOH��Һ�����ȣ����������������� |
| C������������ˮ�ܽ⣬�������ữ���ټ���BaCl2�۲����������� |
| D������������ˮ�ܽ⣬���������Һ������Һ������ɫ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ba(NO3)2 | B��Ba(OH)2 | C��Na2SiO3 | D��Na2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
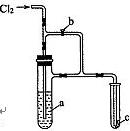
| A��ŨKI��Һ | B��ŨH2SO4 |
| C������ʳ��ˮ | D��NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��
______________________________��Ư�۵���Ч�ɷ��ǣ��ѧʽ��  ����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________
����ܷ������з�Ӧ��Ca��ClO��2+4HCl��Ũ��=CaCl2+2Cl2��+2H2O���÷�Ӧ��ת�Ƶĵ�����Ϊ___________| A���٢ڢ� | B���ڢۢ� | C���ڢ� | D���٢� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com