
ij��ϼ��Ǣ�Na2CO3��NaHCO3 ��� Na2CO3�� NaOH���á� ˫ָʾ����������Ʒ����ֺ��ܼ��� [n (Na2O) ] ���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL ��ƿ�У�����������ˮ�ͼ��η�̪����0.0100 mol??L��1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ����������̡���������Ϊָʾ�����еζ�����NaHCO3��ȫ�к͡�
��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
| ��� | ��һ�ζ��յ�Ķ�����V1/mL�� | �ڶ��ζ��յ�Ķ�����V2/mL�� |
| I | �� | 26.85 |
| II | 10.02 | 25.02 |
| III | 9.98 | 24.98 |
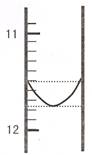
��2���û�ϼ���Ʒ�����Ϊ ������ ��
��3������ϼ�ζ���NaHCO3�Ĺ����У�Ϊ�˽������ȷ��Ӧʼ����εμӣ�����������ԭ���� ��
��4�������������ܼ���[n (Na2O) ]�Ƕ��٣�д���������
��
��5�������ʵ��ֻ�ⶨ��Ʒ���ܼ�����ʵ�鷽��Ӧ��������ƣ�
��
��������
��1��11��76~11��78���÷� �� ��2��Na2CO3 ��NaHCO3 ����Ϊ��һ�ζ��յ��������С�ڵڶ��ζ��յ����������V1<��V2-V1�������˵��
��3���Է��ζ�����ʹNa2CO3ֱ������H2CO3
��4���������ܼ���Ϊ��
n(Na2O)= [��25��02mL +24��98 mL��/2]��10-3L/ mL��0.0100mol/L��1/2��20=0.0025mol ������һ�����ݲ���ȡ�ã����÷֣��ȼ���̼���ƺ�̼�����Ƶ����ʵ�����Ȼ������ܼ�����ֻҪ������ȷҲ���֣�
��5��ֱ���������-�����ƻ����Ϊָʾ���ζ����յ� ��


 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ϼ��Ǣ�Na2CO3��NaHCO3 ���Na2CO3�� NaOH���á�˫ָʾ����������Ʒ����ֺ��ܼ���[n��Na2O��]���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL ��ƿ�У�����������ˮ�ͼ��η�̪����0.0100mol?L-1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ�����������--������Ϊָʾ�����еζ�����NaHCO3��ȫ�кͣ�
ij��ϼ��Ǣ�Na2CO3��NaHCO3 ���Na2CO3�� NaOH���á�˫ָʾ����������Ʒ����ֺ��ܼ���[n��Na2O��]���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL ��ƿ�У�����������ˮ�ͼ��η�̪����0.0100mol?L-1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ�����������--������Ϊָʾ�����еζ�����NaHCO3��ȫ�кͣ�| ��� | ��һ�ζ��յ�Ķ�����V1/mL�� | �ڶ��ζ��յ�Ķ�����V2/mL�� |
| I | �� | 26.85 |
| II | 10.02 | 25.02 |
| III | 9.98 | 24.98 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ϼ��Ǣ�Na2CO3��NaHCO3 ��� Na2CO3�� NaOH���á� ˫ָʾ����������Ʒ����ֺ��ܼ��� [n (Na2O) ] ���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL ��ƿ�У�����������ˮ�ͼ��η�̪����0.0100 mol•L��1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ����������̨D�D������Ϊָʾ�����еζ�����NaHCO3��ȫ�к͡�
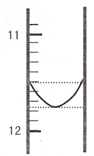
��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
| ��� | ��һ�ζ��յ�Ķ�����V1/mL�� | �ڶ��ζ��յ�Ķ�����V2/mL�� |
| I | �� | 26.85 |
| II | 10.02 | 25.02 |
| III | 9.98 | 24.98 |
��2���û�ϼ���Ʒ�����Ϊ ������ ��
��3������ϼ�ζ���NaHCO3�Ĺ����У�Ϊ�˽������ȷ��Ӧʼ����εμӣ�����������ԭ���� ��
��4�������������ܼ���[n (Na2O) ]�Ƕ��٣�д���������
��
��5�������ʵ��ֻ�ⶨ��Ʒ���ܼ�����ʵ�鷽��Ӧ��������ƣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12 �� )ij��ϼ��Ǣ�Na2CO3��NaHCO3 ��� Na2CO3�� NaOH���á� ˫ָʾ����������Ʒ����ֺ��ܼ��� [n (Na2O)] ���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL��ƿ�У�����������ˮ�ͼ��η�̪����0.0100 mol•L��1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ����������̡���������Ϊָʾ�����еζ�����NaHCO3��ȫ�к͡�
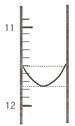
 ��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
| ��� | ��һ�ζ��յ�Ķ�����V1/mL�� | �ڶ��ζ��յ�Ķ�����V2/mL�� |
| I | �� | 26.85 |
| II | 10.02 | 25.02 |
| III | 9.98 | 24.98 |
��2���û�ϼ���Ʒ�����Ϊ ����
�� ��
��3������ϼ�ζ���NaHCO3�Ĺ����У�Ϊ�˽������ȷ��Ӧʼ����εμӣ�����������ԭ���� ��
��4�������������ܼ���[n (Na2O) ]�Ƕ��٣�д���������
��
��5�������ʵ��ֻ�ⶨ��Ʒ���ܼ�����ʵ�鷽��Ӧ��������ƣ�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�廪ʵ��ѧУ2010�������ѧ��3���¿� ���ͣ������
(12 �� )ij��ϼ��Ǣ�Na2CO3��NaHCO3 ��� Na2CO3�� NaOH���á� ˫ָʾ����������Ʒ����ֺ��ܼ��� [n (Na2O) ] ���вⶨ��ʵ�鷽������ȡ 0.2960g ��Ʒ��� 500mL ��Һ��ȡ25.00mL����250mL ��ƿ�У�����������ˮ�ͼ��η�̪����0.0100 mol•L��1 ���������Һ����ϼ�ζ���NaHCO3��Ȼ����������̡���������Ϊָʾ�����еζ�����NaHCO3��ȫ�к͡�
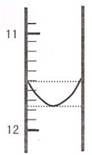 ��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
��1��ijͬѧƽ�еζ������Σ�ÿ�εζ��� "0" �㿪ʼ���������е� I �εζ��ĵ�һ���յ��Һ������ͼ��ʾ�������ʵ�����ݣ��������б�����
|
��� |
��һ�ζ��յ�Ķ�����V1/mL�� |
�ڶ��ζ��յ�Ķ�����V2/mL�� |
|
I |
�� |
26.85 |
|
II |
10.02 |
25.02 |
|
III |
9.98 |
24.98 |
��2���û�ϼ���Ʒ�����Ϊ ����
�� ��
��3������ϼ�ζ���NaHCO3�Ĺ����У�Ϊ�˽������ȷ��Ӧʼ����εμӣ�����������ԭ���� ��
��4�������������ܼ���[n (Na2O) ]�Ƕ��٣�д���������
��
��5�������ʵ��ֻ�ⶨ��Ʒ���ܼ�����ʵ�鷽��Ӧ��������ƣ�
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com