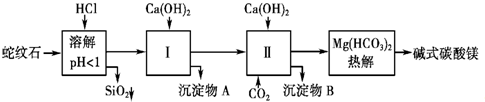
����⣺
��1������ʯ�������ܽ⣬MgO��Fe
2O
3��Al
2O
3��HCl��Ӧ�ܽ⣬��Ӧ����ʽ�ֱ�Ϊ��MgO+2HCl=MgCl
2+H
2O��Fe
2O
3+6HCl=2FeCl
3+3H
2O��Al
2O
3+6HCl=2AlCl
3+3H
2O����SiO
2�������ᷴӦ���Գ�������ʽ��ȥ���ʴ�Ϊ��Fe
3+��Al
3+��
��2���ɣ�1��֪����ʱ��Һ�г���Mg
2+�⣬������Fe
3+��Al
3+���ʣ����ȥFe
3+��Al
3+��������ʧMg
2+����Fe
3++3H
2O?Fe��OH��
3+3H
+��Al
3++3H
2O?Al��OH��
3+3H
+������ƽ���ƶ���ԭ��������H
+ʹ����ˮ��ƽ��������Ӧ�����ƶ�����ȥFe
3+��Al
3+����Ca��OH��
2�轫��Һ��pH������7��8����pH���ߣ��ᵼ�����ɵ�Al��OH��
3������ӦAl��OH��
3+OH-=AlO
2-+2H
2O�ܽ⣬Mg��OH��
2��pHΪ9.4ʱ��ʼ���������Լ�����ǿMg��OH��
2�������ͬʱMg
2+Ҳ��ת��Ϊ��������ʧ��
�ʴ�Ϊ��Al��OH��
3��Mg��OH��
2��
��3����ʵ�鲽��ͼ֪�����������ΪFe��OH��
3��Al��OH��
3����ɫ�������dz�����Fe��OH��
3�ֽ��õ���Fe
2O
3�����Ե��ȼӼ��Al��OH��
3��������ӦΪ��Al��OH��
3+NaOH=NaAlO
2+2H
2O��Ȼ�����ϴ�����ռ��ɣ�
�ʴ�Ϊ��NaOH��Ca��OH��
2�����ˡ�ϴ�ӡ����գ�
��4����ʵ�鲽��ͼ֪������ʵ���У��������ͨ������̼������̼��ƣ�̼��Ʒֽ�ɵõ�������̼����Ϊ�ڢ�ԭ�ϣ���ʽ̼��þ�ֽ�õ�CO
2������ѭ��ʹ�õ�������CaCO
3��CO
2���ʴ�Ϊ��CaCO
3��CO
2��
��5������Ũ�������ˮ�Ը���۹ʴ�Ϊ����Ũ��������ˮ������
���ü�ʯ������CO
2ǰ����������������̼�����ܹʴ�Ϊ��������ʯ������CO
2ǰ���������
��6��m����Ʒ��=18.2g��m��CO
2��=6.6g��m��MgO��=8.0g����ʽ̼��þ�ֽ⣺aMgCO
3?bMg��OH��
2?cH
2O
��a+b��MgO+aCO
2��+��b+c��H
2O�������������غ�ã�m��H
2O��=18.2g-6.6g-8.0g=3.6g����m��MgO���T0.2mol��n��CO
2���T0.15mol��n��H
2O���T0.2mol���ã�a��b��c=0.15��0.05��0.15=3��1��3���ʴ�Ϊ��3��1��3��



 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�