
| ||
| m |
| M |
| 3.6g |
| 18g/mol |
| ||
| 28g��1.6g |
| 16g |
| 2.8g |
| 28g/mol |
| 4.4g |
| 44g/mol |
| 7.6g |
| 76g/mol |
| 0.1mol+0.1mol |
| 0.1mol |
| 0.2mol��2 |
| 0.1mol |
| 0.3mol |
| 0.1mol |
 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ |
| B��12g���ʯ�У���C-C���ۼ�����Ϊ4mol |
| C�����Ӿ������ۻ�ʱ�����Ӽ����ƻ��������Ӿ����ۻ�ʱ����ѧ�������ƻ� |
| D���۵��ɸߵ��͵�˳���ǣ�����裾̼���裾���ʯ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4.6g���ΪC2H6O���л������C-H����Ŀһ��Ϊ0.6NA |
| B��12g���ʯ�к��еĹ��ۼ���Ϊ2NA |
| C��0.1mol�İ��ף�P4��������������ĦҼ�����Ϊ0.4NA |
D��0.5mol�ۻƣ�As4S4�ṹ��ͼ������NA��S-S��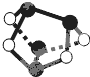 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A����±����RCH2CH2X�л�ѧ����ͼ ��ʾ��������ȥ��Ӧʱ�����ƻ��ļ��Ǣٺ͢� ��ʾ��������ȥ��Ӧʱ�����ƻ��ļ��Ǣٺ͢� |
| B�������������������������Һ���Ҵ����ͻ������ɲ��÷�Һ���� |
C���л���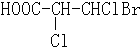 �к���3������̼ԭ�� �к���3������̼ԭ�� |
D�� ��һ������Ҳ�������ʳ�Ϊ��ʽ���飮��ͼ�����ֳ�ʽ���飬���ೲʽ�����ͨʽ��CnHn+4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ͨ�������ۺ��������Ļ��������ȼ� |
| B��ij������ֻ����һ��Ԫ�أ�������һ���Ǵ����� |
| C��40K��40Ca����������ȡ������������ |
| D����ϩ������̼̼˫���ļ��������������̼̼�������ܵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
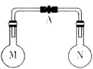 ����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������
����ʱ�������ݻ���ͬ����ƿ�зֱ�ʢ��M��N�������壨ͬ��ͬѹ����ȡ�µ��ɼ�A��ʹ����ƿ�ڵ�����Ӵ�����ͼ���������ڵ�ѹǿ�ɴ�С��˳���ǣ�������| ��� | �� | �� | �� | �� |
| ����M | H2S | H2 | NH3 | NO |
| ����N | SO2 | Cl2 | HCl | O2 |
| A���٢ڢۢ� | B���٢ܢۢ� |
| C���ڢܢ٢� | D���ܢ٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ݢܢ٢� | B���ݢ٢ܢڢ� |
| C���ܢ٢ڢݢ� | D���ۢڢܢ٢� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com