

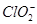 ��O2����2H2O
��O2����2H2O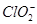 ��3Cl����4H��=2Cl2����2H2O
��3Cl����4H��=2Cl2����2H2O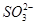 �����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H����
�����������¾��н�ǿ�Ļ�ԭ�ԣ��ʢ��з�����Ӧ����������NaClO3����ԭ����Na2SO3��(2)���Na2SO4��Һ�൱�ڵ��ˮ��������a��b��Na2SO4��Һ�ĵ������֪ΪH2��O2��������aΪ��Ӧ��IJ���֮һ��֪��ΪO2��ΪH2O2��ClO2�����õ��IJ���ʷ�Ӧ��ķ�Ӧ��ΪClO2��H2O2��NaOH����������NaClO2��O2�����ݵ����غ��ԭ���غ���ƽ���ɣ�(3)���Na2SO4��ҺʱOH�����������ɣ���Na��ͨ�����Ӹ�Ĥ�������ƶ��õ�����NaOH��Һ������������H���� �������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��
�������ƶ��õ�����A(��H2SO4��Һ)��(4)���������֪��Ӧ��ΪNaClO2��HCl������NaClO2��ClԪ�صĻ��ϼ�Ϊ��3�ۣ��ȿ�����Ҳ�ɽ��ͣ��ʷ�ӦΪNaClO2������������ԭ��Ӧ�����ݵ����غ��֪��������ClO2�ͻ�ԭ����Cl�������ʵ���֮��Ϊ4��1������ԭ���غ㽫����ʽ��ƽ���ɣ���������ΪCl2��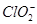 ��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��
��HCl�����˹��з�Ӧ�����ݵ����غ��֪��Ӧ��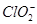 ��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�
��HCl�����ʵ���֮��Ϊ3��1������ԭ���غ㽫����ʽ��ƽ���ɣ�(5)���ʹ���ΪNaClO2������������ԭ��Ӧ�����۱��������Fe2����Ӧʱ��Ԫ�����ն�ת��ΪCl������ת�Ƶ�������ȡ�

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A����ˮ�д��������µ�ƽ�⣺Br2��H2O HBr��HBrO����Ҫʹ��ˮ����ɫ��dz���ɲ�ȡ�Ĵ�ʩ�Ǽ���NaI���� HBr��HBrO����Ҫʹ��ˮ����ɫ��dz���ɲ�ȡ�Ĵ�ʩ�Ǽ���NaI���� |
| B������ˮƿ�м���Na2CO3��Һ�ݽ�����������ܳ�ȥ�ڱ�CaSO4 |
| C���ñ��͵�NaOH����Һ��ȥ���������е��Ҵ������� |
| D���������ƾ��н�ǿ�Ļ�ԭ�ԣ������������������Ƿ���ʣ� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��K����SiO32-��Cl����NO3- |
| B��H����NH4+��Al3����SO42- |
| C��Na����Cl����MnO4-��SO42- |
| D��Na����CO32-��CH3COO����HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
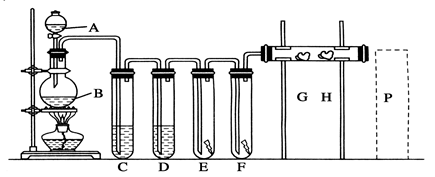
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
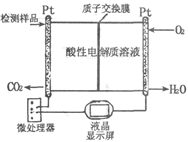
| A����ϵѹǿ���ֲ��� | B�����������ɫ���ֲ��� |
| C��SO3��NO������ȱ��ֲ��� | D��ÿ���� 1 mol SO2��ͬʱ����1 mol NO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
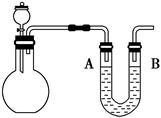
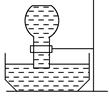
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 10-2mol��L-1��II�з�Ӧ����NaClO2��Һ(������NaOH)��pH=13������Һ��
10-2mol��L-1��II�з�Ӧ����NaClO2��Һ(������NaOH)��pH=13������Һ��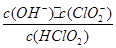 = ��
= ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com