
(10��)����ͼ��ʾ�ķ�Ӧ��ϵ��A����ѧ���������A��B��C�к���ͬһ��Ԫ��R������R��Ԫ���Ѿ���ȥ��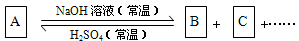
(1)��A��NaOH����ͬ���ʵ����ȷ�Ӧ���ȿ�ֻ����B���ֿ�ֻ����C����������B+C��
��д������������Ӧ��ϵ��A�Ļ�ѧʽ ��
��д�������йط�Ӧ�����ӷ���ʽ��
����B�����ӷ���ʽΪ ��
����C�����ӷ���ʽΪ ��
��2����A��NaOH�����Ժ������ʵ����ȷ�Ӧ��������ΪB+C����A�ķ���ʽ������
��ֻдһ�֣����÷�Ӧ�����ӷ���ʽΪ ��
R��A��B��C�еĻ��ϼ۱�������������� ��
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)����ͼ��ʾ�ķ�Ӧ��ϵ��A����ѧ���������A��B��C�к���ͬһ��Ԫ��R������R��Ԫ���Ѿ���ȥ��
(1)��A��NaOH����ͬ���ʵ����ȷ�Ӧ���ȿ�ֻ����B���ֿ�ֻ����C����������B+C��
��д������������Ӧ��ϵ��A�Ļ�ѧʽ ��
��д�������йط�Ӧ�����ӷ���ʽ��
����B�����ӷ���ʽΪ ��
����C�����ӷ���ʽΪ ��
��2����A��NaOH�����Ժ������ʵ����ȷ�Ӧ��������ΪB+C����A�ķ���ʽ������
��ֻдһ�֣����÷�Ӧ�����ӷ���ʽΪ ��
R��A��B��C�еĻ��ϼ۱�������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪C���ܶ���С�����壬���ǵ���ʡ�
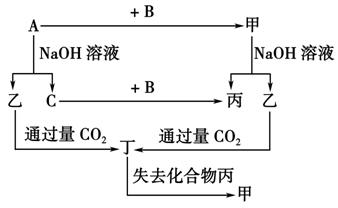
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��
A________��B________����________����________��
(2)��ɵ���A��Ԫ�������ڱ��е�λ����_____________�����ĵ���ʽ��
(3)д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��__________________________________________
���������CO2��Ӧ�����ӷ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ɹŰ������и������п��Ի�ѧ�Ծ� ���ͣ������
(10��)����ͼ��ʾ�ķ�Ӧ��ϵ��A����ѧ���������A��B��C�к���ͬһ��Ԫ��R������R��Ԫ���Ѿ���ȥ��
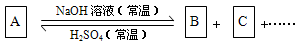
(1)��A��NaOH����ͬ���ʵ����ȷ�Ӧ���ȿ�ֻ����B���ֿ�ֻ����C����������B+C��
��д������������Ӧ��ϵ��A�Ļ�ѧʽ ��
��д�������йط�Ӧ�����ӷ���ʽ��
����B�����ӷ���ʽΪ ��
����C�����ӷ���ʽΪ ��
��2����A��NaOH�����Ժ������ʵ����ȷ�Ӧ��������ΪB+C����A�ķ���ʽ������
��ֻдһ�֣����÷�Ӧ�����ӷ���ʽΪ ��
R��A��B��C�еĻ��ϼ۱�������������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ֣������ʮ���и�����ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(10��)�ɶ�����Ԫ����ɵĵ���A��B��C�ͼס��ҡ����������ֻ���������ͼ��ʾ��ת����ϵ����֪C���ܶ���С�����壬���ǵ���ʡ�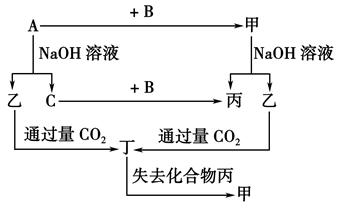
����ͼʾת����ϵ�ش�
(1)д���������ʵĻ�ѧʽ��
A________��B________����________����________��
(2)��ɵ���A��Ԫ�������ڱ��е�λ����_____________�����ĵ���ʽ��
(3)д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________ ___________________________
___________________________
���������CO2��Ӧ�����ӷ���ʽ��__________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com