
�������������ش�1��~��3���⡣
��һ���ݻ��̶��ķ�Ӧ������һ�����һ������ܷ���壬����ֱ������ͼ��ʾ�Ŀ��淴Ӧ��
2A(g)+B(g)=2C(g) | D(g)+3E(g)=2F(g) |
�����ʵ���ʼ���������£�A��B��C��D��E��F�����ʵ����ֱ�Ϊ2mol��1mol��0 mol��Xmol��Ymol��Zmol�����߷�Ӧ��ƽ�����ǡ�������м��λ�á�
��1������ʱ��������������г���0.3mol He(He����A��B��C��D��E��F��Ӧ)������˵����ȷ����
A��A�����ʵ������ӡ� �������������������������� B��B��ת��������
C��C�����ʵ������䡡 �������������������������� D��F��Ũ�ȣ��������һ��������
��2������ʱ���������������ͨ�˵IJ���He������2 molA��1mol B�����壬������˵����ȷ����
�� A��A�������������
�� B��B��ת���ʽ���
�� C��C�����������Ũ�ȶ�����
�� D���ұ�����������ƽ����Է�����������
��3������ʱ�ڣ�2�����ƽ����ϵ�У�����������������ͬʱ�ٸ�ע��lmolHe��ƽ����루2�����ƽ����ϵ��ȣ�����˵����ȷ����
�� A��C��F��Ũ�ȶ�����
�� B��B��ת���ʣ�F�ķֽ��ʶ�������
�� C��A��D���ʵ�����������
�� D��A��B��C��D��E��F���ʵ���������
 ״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
״Ԫ��ȫ��ͻ�Ƶ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)��֪���ڵĽṹ��ʽΪ��
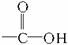
�ٺϳɵ��ڵĵ�����__________��__________��__________(�ɲ�������Ҳ�ɲ���)��
�ںϳɵ��ڵķ�Ӧ����ʽ�ͷ�Ӧ���ͷֱ���____________��____________��
(2)��֪�ϳɽ���ֻ����һ��ԭ�ϣ�����ԭ�ϵĽṹ��ʽ�� 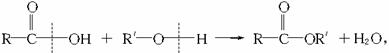
��ϳɽ��ڵķ�Ӧ����ʽ�ͷ�Ӧ������_____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪���ں͵����dz��õIJ��ϡ���������������ش��й����⡣
(1)��֪���ڵĽṹ��ʽΪ��
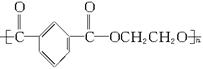
�ٺϳɵ��ڵĵ�����__________��__________��__________(�ɲ�������Ҳ�ɲ���)��
�ںϳɵ��ڵķ�Ӧ����ʽ�ͷ�Ӧ���ͷֱ���____________��____________��
(2)��֪�ϳɽ���ֻ����һ��ԭ�ϣ�����ԭ�ϵĽṹ��ʽ��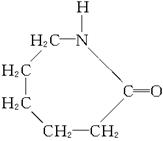
��ϳɽ��ڵķ�Ӧ����ʽ�ͷ�Ӧ������_____________��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ��������ľ��ѧ�߶���һ���¿���ѧ�Ծ����������� ���ͣ������
��11�֣���Դ�ɻ���Ϊһ����Դ�Ͷ�����Դ����Ȼ�������ֳ���ʽ�ṩ����Դ��Ϊһ����Դ��������������Դ�����������ȡ����Դ��Ϊ������Դ��������һ�ָ�Ч��û����Ⱦ�Ķ�����Դ������������Ȼ���д������ڵ�ˮ����ȡ��2H2O(l)===2H2(g)��O2(g)����H����571.6 kJ/mol���������������ش�
(1)����������ȷ���� ����������ѡ��(4��)
A�������Ƕ�����Դ�������� B��ˮ���Ƕ�����Դ
C����Ȼ����һ����Դ D����¯����һ����Դ
(2)��֪��CH4(g)��2O2(g)===CO2(g)��2H2O(l)����H����890.3 kJ/mol���������������ͼ���ֱ���ȫȼ�պų�������֮��ԼΪ(����)��3�֣�
A��1��3.4 B��1��1.7 C��2.6��1 D��4.6��1
(3)������ˮ��ȡ������Դ�����������о�������ȷ���� ����������ѡ��4�֣�
A�����ˮ����������ǿ���ȼ�յ����ʣ���˿��о���ˮ���ֽ������£�ʹ���Ϊ������Դ
B���跨��̫����۽����������£�ʹˮ�ֽ��������
C��Ѱ�Ҹ�Ч������ʹˮ�ֽ����������ͬʱ�ͷ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com