
��������FeCl3��Һ�μӵķ�Ӧ�У���Fe3+ˮ���йصķ�Ӧ��
��FeCl3��Һ��Cu�ķ�Ӧ �ڽ�FeCl3��Һ�������ɣ����������յõ�Fe2O3 ��FeCl3��Һ��KI�ķ�Ӧ �ܱ���FeCl3��Һ�����ˮ���Ʊ�Fe(OH)3���� ��FeCl3��Һ��H2S�ķ�Ӧ��FeCl3��Һ��NaHCO3��Һ�ķ�Ӧ ������FeCl3��Һ�����һ����������
| A���٢ܢ� | B���ڢۢݢ� | C���ڢܢޢ� | D���٢ڢۢܢݢޢ� |
C
������ȷ�𰸣�C
ˮ�ⷽ��Ϊ;Fe3����3H2O Fe(OH)3��3H��
Fe(OH)3��3H��
����Fe3+ˮ���أ�FeCl3�������� ����Fe3+ˮ���йأ���FeCl3��Һ���ȣ�HCl�ӷ���Fe(OH)3���պ�ֽ⣬���յõ�Fe2O3 ����Fe3+ˮ���أ�FeCl3�������� ����Fe3+ˮ���йأ�����FeCl3��Һ�����ˮ��ˮ�⣬�Ʊ�Fe(OH)3���� ����Fe3+ˮ���أ�FeCl3��Һ������������Fe3+ˮ���йأ�FeCl3ˮ������ԣ�NaHCO3ˮ��ʼ��ԣ���ٽ� ����Fe3+ˮ���йأ�����FeCl3��Һ�����һ���������ᣬ����ˮ�⣬��ֹ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
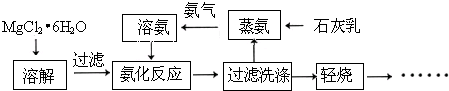
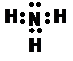
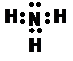
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����4�֣������й�ʵ�����������˵������ȷ����
A����pH��ֽ������ˮʪ�����ij��Һ��pH
B����ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ�����������
C���ζ���ϴ��������ˮ��ϴ����ע���Һ�����к͵ζ�ʵ��
D�����������Һ���������������ͨ��һ����HCl����ʱ���������Һ�ɻָ�����ԭ��һ��
E���ù㷺pH��ֽ����Na2S��Һ��pHʱ����pH=10.5
F��ʵ����������FeCl3��Һʱ���Ƚ�FeCl3����һ������Ũ�����У��ټ�����ˮϡ��������Ũ��
��2����6�֣�ijͬѧ�ڰ�����ʦ����ʵ���ҵĻ�ѧ�Լ�ʱ������һʢ����ɫ��Һ���Լ�ƿ����ǩ����(����ͼ)��������������յ�֪ʶ���Ը��Լ�������ʲô���ʵ���Һ�������룬�����ʵ�������֤��
�ٲ��룺�����Լ�������_____________________��
�ڼ�����֤��ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����صڶ�����ѧ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
��1����4�֣������й�ʵ�����������˵������ȷ����
| A����pH��ֽ������ˮʪ�����ij��Һ��pH |
| B����ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ����������� |
| C���ζ���ϴ��������ˮ��ϴ����ע���Һ�����к͵ζ�ʵ�� |
| D�����������Һ���������������ͨ��һ����HCl����ʱ���������Һ�ɻָ�����ԭ��һ�� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꺣��ʡ�߶���ѧ����ĩ���Ի�ѧ���������� ���ͣ�ʵ����
��6�֣������й�ʵ�����������˵������ȷ���� ���ڸ�����ѡ���У������������Ƿ�����ĿҪ��ģ�
A����pH��ֽ������ˮʪ�����ij��Һ��pH
B����ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ�����������
C���ζ���ϴ��������ˮ��ϴ����ע���Һ�����к͵ζ�ʵ��
D�����������Һ���������������ͨ��һ����HCl����ʱ���������Һ�ɻָ�����ԭ��һ��
E���ù㷺pH��ֽ����Na2S��Һ��pHʱ����pH=10.5
F��ʵ����������FeCl3��Һʱ���Ƚ�FeCl3����һ������Ũ�����У��ټ�����ˮϡ��������Ũ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ����ĩ���Ի�ѧ���� ���ͣ�ʵ����
��1����4�֣������й�ʵ�����������˵������ȷ����
A����pH��ֽ������ˮʪ�����ij��Һ��pH
B����ͭƬ����Ƭ������һ�����ϡ�����У�ͭƬ�����������
C���ζ���ϴ��������ˮ��ϴ����ע���Һ�����к͵ζ�ʵ��
D�����������Һ���������������ͨ��һ����HCl����ʱ���������Һ�ɻָ�����ԭ��һ��
E���ù㷺pH��ֽ����Na2S��Һ��pHʱ����pH=10.5
F��ʵ����������FeCl3��Һʱ���Ƚ�FeCl3����һ������Ũ�����У��ټ�����ˮϡ��������Ũ��
��2����6�֣�ijͬѧ�ڰ�����ʦ����ʵ���ҵĻ�ѧ�Լ�ʱ������һʢ����ɫ��Һ���Լ�ƿ����ǩ����(����ͼ)��������������յ�֪ʶ���Ը��Լ�������ʲô���ʵ���Һ�������룬�����ʵ�������֤��

�ٲ��룺�����Լ�������_____________________��
�ڼ�����֤��ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com