
��ѧ�ϳ���ȼ�շ�ȷ���л������ɣ���ͼװ������ȼ�շ�ȷ���л��ﻯѧʽ���õ�װ�ã����ַ������ڵ�¯����ʱ�ô�������������Ʒ�����ݲ��������ȷ���л������ɣ�

�ش��������⣺
(1)Aװ���з�Һ©��ʢ�ŵ�������______________��д���йط�Ӧ�Ļ�ѧ����ʽ
______________________________________________________________��
(2)Cװ��(ȼ�չ�)��CuO��������______________________________________
____________________________.
(3)д��Eװ������ʢ���Լ�������__________������������______________��
(4)����Bװ��ȥ�����ʵ�����ʲôӰ�죿 __________________________.
(5)��ȷ��ȡ1.20 g��Ʒ(ֻ��C��H��O����Ԫ���е����ֻ�����)�������ȼ�պ�
E����������1.76 g��D����������0.72 g������л�������ʽΪ______________��
(6)Ҫȷ�����л���Ļ�ѧʽ������Ҫ�ⶨ________________________��
(1)H2O2(��˫��ˮ)2H2O2 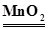 2H2O��O2��(��H2O��2Na2O2��2H2O===4NaOH��O2��)
2H2O��O2��(��H2O��2Na2O2��2H2O===4NaOH��O2��)
(2)ʹ�л�������������CO2��H2O (3)��ʯ�һ��������ơ�����CO2
(4)��ɲ���л����к���������
(5)CH2O ��(6)����л������Է�������
��������
�����������ʵ��ʹ��ȼ�շ��ⶨ�л�����ɣ���ʵ��װ�ð��ա�������������������ȼ���л��������ˮ�����ն�����̼�����С�ʵ��ɲ�֪ȼ�����ɵĶ�����̼��ˮ�����������ݶ�����̼����������CԪ�ص���������ˮ�����������HԪ�ص�����������л�������������OԪ�ص��������ɴ˼���ȷ���л��������C��H��O�����ȣ�Ҳ����ȷ����ʵ��ʽ����Ҫ�ٽ�һ��ȷ���л���ķ���ʽ������֪�����л������Է���������
��1��װ��A���Ʊ������ģ����Ը���װ�õ��ص��֪Aװ���з�Һ©��ʢ�ŵ�������˫��ˮ��ˮ��Ӧ�÷�Ӧ�Ļ�ѧ����ʽ��2H2O2 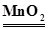 2H2O��O2����2Na2O2��2H2O===4NaOH��O2����
2H2O��O2����2Na2O2��2H2O===4NaOH��O2����
��2�������л�����ȼ�չ����У��п��ܲ���CO������Cװ��(ȼ�չ�)��CuO��������ʹ�л�������������CO2��H2O��
��3�������л���ȼ�ղ���CO2������Eװ�õ���Ҫ�������������ɵ�CO2�����������ʢ���Լ��Ǽ�ʯ�һ��������ơ�
��4��Bװ�õ������Ǹ�����������ȥˮ��������������Bװ��ȥ������ɲ���л����к���������
��5��D����������0.72 g�������ɵ�ˮ��0.72g�����ʵ�����0.04mol��������Ԫ�ص�������0.08g��E����������1.76 g����CO2��1.76g�����ʵ�����0.04mol������̼Ԫ�ص�������0.48g����ԭ�л�������Ԫ�ص�������1.20g��0.08g��0.48g��0.64g�����ʵ�����0.04mol������ԭ�л�����C��H��O��ԭ�Ӹ���֮����1:2:1�������ʽ��CH2O��
��6������֪���ʽ������£�Ҫȷ���û�����Ļ�ѧʽ������Ҫ����л������Է���������
���㣺��������л���ȼ�շ�ȷ���л��ﻯѧʽ�ļ��㡢�ж��Լ�ʵ�����
�������������е��Ѷȵ����⣬�����ۺ���ǿ���������У������߿�����Ҫ�ǿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������ͷ�ɢ˼ά����������ѧ����Ҫ��ȷ���Ǹ��������ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

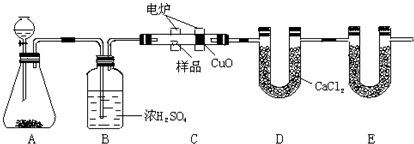
| MnO2 |
| MnO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com