
��1����5�֣�
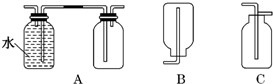
��ͼ�е�װ�ÿ�������ȡ��Ȳ����ش�ͼ��A�ܵ�����_______________
��ȡ��Ȳ�ķ���ʽ�ǣ�___________________________��
Ϊ���ⷴӦ̫Ѹ�٣��ɲ�ȡ�Ĵ�ʩ��______________________��

��2����7�֣�1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g・mL���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ��_________________________ �� __________________________ ��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е���__________________________
������c ��NaOH��Һ�������� ________________________
��1����5�֣�����ˮ��߶��Կ��Ʒ�Ӧ�ķ�����ֹͣ��2�֣���
CaC2+2H2O��Ca (OH)2+C2H2����2�֣� ����Ȳ��д�ṹ��ʽ��
�ñ���ʳ��ˮ����ˮ��1�֣�
��2����7�֣�CH3CH2OH �� CH2��CH2����H2O��2�֣���������Ũ���ᣬ���ȣ�CH2��CH2��Br2 �� CH2BrCH2Br��2�֣�
��3��b��ˮ���½�����������ˮ�����������������2�֣�
��4�� ��ȥ��ϩ�е��������壨SO2��CO2����1�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


| ||
| �� |
| ||
| �� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ�ʵ����
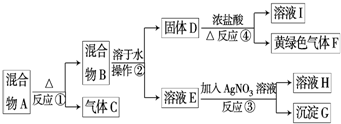
��14�֣��ӹ�������A�������Է������¿�ͼ��ʾ��һϵ�б仯��
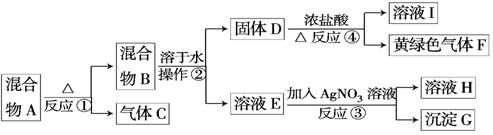
GΪ������ϡ����İ�ɫ���壻��ҺH����ɫ��Ӧ����ɫ(����ɫ���ܲ����۲�)��
�ش��������⣺
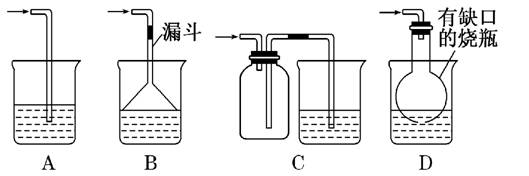
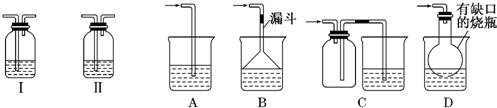
(1)��ʵ�������ռ�����C����ѡ����ͼװ���е�____ ____��
(2)�����ڵ�������___ ___���ڲ���������ʹ�õIJ���������������_____ ��
(3)д����Ӧ�ܵ����ӷ���ʽ�� _______________ _________________________��
(4)��ʵ�����У�Ҫ��ø��﴿���Ļ���ɫ����F�����Խ���ͨ����ͼ�е�װ�ã�����ƿ����ʢ�ŵ���______ ___��ƿ����ʢ�ŵ���____________ _____��
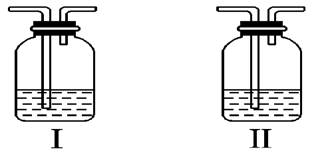
(5)ʵ���б������ն��������F��������Ⱦ��������ͼ��ʵ������NaOH��Һ��������F��װ�ã�Ϊ��ֹ����������������װ����________ ____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����5�֣�
��ͼ�е�װ�ÿ�������ȡ��Ȳ����ش�ͼ��A�ܵ�����_______________
��ȡ��Ȳ�ķ���ʽ�ǣ�___________________________��
Ϊ���ⷴӦ̫Ѹ�٣��ɲ�ȡ�Ĵ�ʩ��______________________��

��2����7�֣�1��2����������������Ϳ����������Ӽ���������������ɫҺ�壬�ܶ�2.18g・mL���е�131.4�棬�۵�9.79�棬������ˮ�������ڴ����ѡ���ͪ���л��ܼ�����ʵ�����п�������ͼ��ʾװ���Ʊ�1��2���������顣���з�Һ©������ƿa ��װ���Ҵ���ŨH2SO4�Ļ��Һ���Թ�d ��װ��Һ�壨���渲������ˮ����

��д���пհף�
��д���������Ʊ�1��2�����������������ѧ����ʽ��_________________________ �� __________________________ ��
�ư�ȫƿb ���Է�ֹ�����������Լ��ʵ�����ʱ�Թ�d �Ƿ�����������д����������ʱƿb �е���__________________________
������c ��NaOH��Һ�������� ________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com