
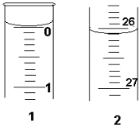
 ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�
ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һʱ��ѡ�������ָʾ��������д���пհף�| �ζ����� | ����NaOH��Һ�����/mL | 0.1000mol/L��������/mL | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ���/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |
���� ��1����������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯�����ж��ζ��յ㣻�ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ԭ��
��2������c�����⣩=$\frac{c��������V������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��3�����ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ����
��4���ȸ������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ�����Ÿ���HCl+NaOH=NaCl+H2O���c��NaOH����
��� �⣺��1������к͵ζ�ʱ���۾�Ҫע����ƿ����Һ����ɫ�仯���ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ԭ��
�ʴ�Ϊ����ƿ����Һ��ɫ�仯��������ڲ���ԭ��
��2��A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ����Һ��ϡ�ͣ����V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫ��A����
B���ζ�ǰʢ������������Һ����ƿ������ˮϴ����û�и������Һ�����ʵ������䣬��V��������Ӱ�죬����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩��Ӱ�죬��B����
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ�����V������ƫС���������V������ƫ����c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫ��C����
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ��������V������ƫС������c�����⣩=$\frac{c��������V������}{V�����⣩}$�������ⶨc�����⣩ƫС����D��ȷ��
��ѡD��
��3����ʼ����Ϊ0.00mL���յ����Ϊ26.10mL��������Һ�����Ϊ26.10mL��
�ʴ�Ϊ��0.00��26.10��26.10��
��4���������ݵ���Ч�ԣ���ȥ��2�����ݣ�Ȼ�����1��3��ƽ������V�����ᣩ=$\frac{26.11+26.09}{2}$mL=26.10mL�����ݷ�Ӧ����ʽ��HCl+NaOH=NaCl+H2O��n��HCl��=n��NaOH��������0.0261L��0.1000mol•L-1=0.025L��c��NaOH�������c��NaOH��=$\frac{0.0261L��0.1000mol/L}{0.025L}$=0.1044mol/L��
�ʴ�Ϊ��0.1044mol/L��
���� ������Ҫ�������к͵ζ�������������ѧ���ķ�����ʵ��ͼ��������Ŀ��飬�ѶȲ���ע���к͵ζ���ԭ��������к�ʱH+�����ʵ�����OH-���ӵ����ʵ�����ȣ�������������


 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
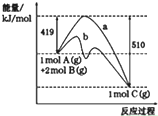
| A�� | ����b��ʾʹ�ô�����������仯 | |
| B�� | ����Ӧ��ܴ����淴Ӧ��� | |
| C�� | ��ͼ��֪�÷�Ӧ���ʱ��H=+91 kJ•mol-1 | |
| D�� | ��Ӧ�н�����A��Ϊ���巴Ӧ�������������䣬��Ӧ�ų���������91kJ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ೱ����ɫ��Ⱦ����ɫʳƷ�еġ��ࡱ���ס����̡���ָ������ʵ���ɫ | |
| B�� | Ϊ��ֹկ����������������Ư�۾���˫��ˮ�Ի����������� | |
| C�� | �����������ÿ�ȼ������̬����ˮ����������ں�����̬���������� | |
| D�� | ��������ҺӦ��������в������Ĺ���Լ�ƿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | ����ʯ��ˮ | C�� | FeSO4 | D�� | Na2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����к͵ζ�ʱ���ô���Һ��ϴ��ƿ | |
| B�� | ����к͵ζ�ʱ���ó�ϴ�ɾ��ĵζ���ʢװ����Һ | |
| C�� | �ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�����������ȷ | |
| D�� | ��ʽ�ζ��ܼ��첿�ֿ�ʼʵ��ʱ�����ݣ��ζ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����=������0ʱ��״̬ | B�� | NO2ȫ��ת���N2O4��״̬ | ||
| C�� | c��NO2��=c��N2O4����״̬ | D�� | ��ϵ����ɫ�����仯��״̬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ӻ������ж��������Ӽ� | |
| B�� | ���ӻ������е�������ֻ���ǽ������� | |
| C�� | ���ӻ�����һ�����Ե��� | |
| D�� | ����ˮ���Ե���Ļ�����һ�������ӻ����� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com