
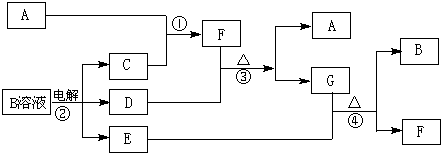
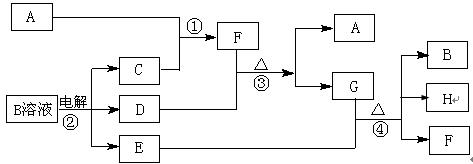
��12�֣���ͼΪ��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ�����ֲ�������ȥ������֪��A��C��D�dz��������嵥�ʣ�F���弫������ˮ����Һ̬�����������
��1��д����ѧʽA ��D ��F ��G���� ���壻
��2������G�������ӵ�ʵ�鷽��������_____________________________��
��3��D���ʺ�E��Һ��Ӧ������һ�ֳ�������������Ư������Ч�ɷ֣�д��D+E��Һ��Ӧ�����ӷ���ʽ�͢۵Ļ�ѧ����ʽ ��
��
��4�������£����B��Һ�Ƶ�pH = 12���ռ���Һ1000mL��Ӧ��ת�Ƶĵ�����ĿΪ ��
 ��Ԫ������ĩ��ϵ�д�
��Ԫ������ĩ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
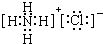
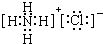
| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�����ʡ��У�����ڶ������������ۣ���ѧ���� ���ͣ������
��15�֣���ͼΪ��ѧ��ѧ�м��ֳ�������֮���ת����ϵ����֪����1��A��C��D�dz��������嵥�ʣ���2��F���弫������ˮ����Һ̬���������������3��GΪ�����Ե��Σ�EΪ��ɫ��������4��HΪ��ɫҺ�廯���
|
 ��1��д��G�ĵ���ʽ ��
��1��д��G�ĵ���ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡТ�и��и���9�µ��п��Ի�ѧ�Ծ����������� ���ͣ������
��10�֣���ͼΪ��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ�����ֲ�������ȥ������֪��A��D�ǽ������ʣ�LΪ���ɫ������EΪʳ�ε���Ҫ�ɷ֣�I��ˮ��Һ��ǿ���ԡ�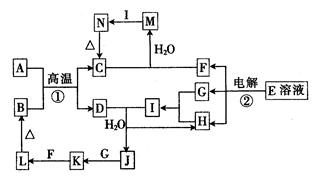
��1��K�Ļ�ѧʽΪ______________________________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ��______________________________________��
��3��д����Ӧ�ڵ����ӷ���ʽ��______________________________________��
��4����M��Һ�м���������I��Һ��Ӧ�����ӷ���ʽΪ______________________��
��5��д��һ���ɻ��Ϸ�Ӧ����L�Ļ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�������в�����ͨ���и�����һ�����Ͽ��Ի�ѧ�Ծ����������� ���ͣ��ƶ���
��ͼΪ��ѧ��ѧ�м��ֳ������ʵ�ת����ϵ�����ֲ�������ȥ������֪��A��D�ǽ������ʣ�LΪ���ɫ������EΪʳ�ε���Ҫ�ɷ֣�I��ˮ��Һ��ǿ���ԡ�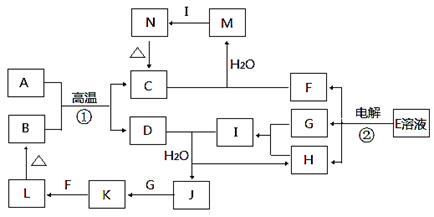
��1��д����Ӧ�ٵĻ�ѧ����ʽ��______________________________________��
��2��д����Ӧ�ڵ����ӷ���ʽ��______________________________________��
��3����M��Һ�м���������I��Һ��Ӧ�����ӷ���ʽΪ______________________��
��4��д��һ���ɻ��Ϸ�Ӧ����L�Ļ�ѧ����ʽ______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com