
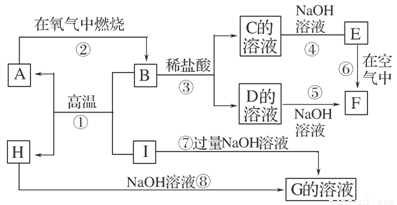
(9��) A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
(1)A��B��C��D��E��F��������������ͬһ��Ԫ����Ԫ�����ڱ���λ��________��
(2)д��C��H���ʵĻ�ѧʽ��C________��H________��
(3)д����Ӧ�٢ߵĻ�ѧ����ʽ��
��Ӧ�٣�______________________________________________________________��
��Ӧ�ߣ�______________________________________________________________��
(4)��Ӧ�����е�������______________________________________________��
(1)�������ڡ��ڢ���
(2)FeCl2��Al2O3
(3)8Al��3Fe3O44Al2O3��9Fe
2Al��2NaOH��2H2O===2NaAlO2��3H2��
(4)���ɵİ�ɫ�����ڿ�����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
������������ͻ�ƿ�ΪF�Ǻ��ɫ������ˮ�ij������������뵽Ϊ��Ϥ��Fe(OH)3������E��ΪFe(OH)2������HΪ����Ԫ�أ���̬����������B��I�ڸ���������Ӧ���ɵIJ������뵽���ȷ�Ӧ�����HΪAl2O3��AΪFe����O2��ȼ������������B��Fe3O4��IΪAl��C��D��G���ѵõ��ֱ�Ϊ��FeCl2��FeCl3��NaAlO2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��) A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
(1)A��B��C��D��E��F��������������ͬһ��Ԫ����Ԫ�����ڱ���λ��________��
(2)д��C��H���ʵĻ�ѧʽ��C________��H________��
(3)д����Ӧ�٢ߵĻ�ѧ����ʽ��
��Ӧ�٣�______________________________________________________________��
��Ӧ�ߣ�______________________________________________________________��
(4)��Ӧ�����е�������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
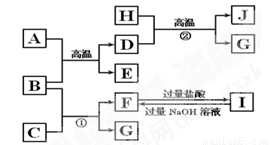
(9��)��ͼ��A��J�������������ˮ��Һ������B��D��G�ǵ��ʣ�A�Ǻ���ɫ��ĩ��G�����壬J�Ǻ�ɫ���塣
����ͼʾ�ش����⣺
��1��д���������ʵĻ�ѧʽ��A ��E ��I ��
��2����Ӧ�ڵĻ�ѧ����ʽ�� ��
��3��J�����ᷴӦ�Ļ�ѧ����ʽ�� ��
���������ʵ���G�ֱ����ĵ���D��B �����ʵ���֮��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
(9��) A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮�����ϵ����ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ����Ԫ�صĹ�̬�����F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհף�
(1)A��B��C��D��E��F��������������ͬһ��Ԫ����Ԫ�����ڱ���λ��________��
(2)д��C��H���ʵĻ�ѧʽ��C________��H________��
(3)д����Ӧ�٢ߵĻ�ѧ����ʽ��
��Ӧ�٣�______________________________________________________________��
��Ӧ�ߣ�______________________________________________________________��
(4)��Ӧ�����е�������______________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(9��)��ͼ��A��J�������������ˮ��Һ������B��D��G�ǵ��ʣ�A�Ǻ���ɫ��ĩ��G�����壬J�Ǻ�ɫ���塣
����ͼʾ�ش����⣺
��1��д���������ʵĻ�ѧʽ��A ��E ��I ��
��2����Ӧ�ڵĻ�ѧ����ʽ�� ��
��3��J�����ᷴӦ�Ļ�ѧ����ʽ�� ��
���������ʵ���G�ֱ����ĵ���D��B �����ʵ���֮��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com