
����Ŀ��Co��Ni�Ļ��������������������Ź㷺��Ӧ�á�
(1)![]() Ԫ�������ڱ��е�λ����4���ڣ�_________�塣��̬Coԭ�ӵĵ����Ų�ʽΪ____________��
Ԫ�������ڱ��е�λ����4���ڣ�_________�塣��̬Coԭ�ӵĵ����Ų�ʽΪ____________��
(2)�Ҷ���NH2-CH2-CH2-NH2����д����en����Nԭ�ӵ��ӻ���ʽΪ________�ӻ���en������Co�γ������[Co(en)2Cl2]Cl��HCl��2H2O�������ӽṹ����ͼ��ʾ���������ӵ���λ��Ϊ________������ᄃ���п��ܴ��ڵ���������___________��
A�����Ӽ� B�����Թ��ۼ� C���Ǽ��Թ��ۼ� D����λ�� E�����

(3)NiO����ṹ��ͼ1��ʾ�����������������AΪ(0,0,0)��BΪ(![]() )����C���ӣ����Ĵ����������Ϊ_______________��
)����C���ӣ����Ĵ����������Ϊ_______________��

(4)��Ȼ���������������Ǵ��ھ���ȱ�ݣ���ͼ2��ʾ��NiXO������xֵΪ![]() ���������е�Ni�ֱ�Ϊ
���������е�Ni�ֱ�Ϊ![]() ��
��![]() ���˾�����
���˾�����![]() ��
��![]() �����������Ϊ______��
�����������Ϊ______��
���𰸡�VIII [Ar]3d74s2 sp3 6 ABCDE ��0��1/2��1/2�� 8:3
��������
��1��COԭ�ӵĺ˵����Ϊ27��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬��̬CO�ĵ����Ų�ʽΪ[Ar]3d74s2��
��2���Ҷ�����H2N-CH2-CH2-NH2����Nԭ�Ӳ�ȡsp3�ӻ��������Ҷ�������������������Ϊ��λ���N������һ���Ҷ��������ṩ������λ����һ������λ������2��2+2=6���������Ļ�ѧʽ��֪��[Co(en)2Cl2]Cl��HCl��2H2O�����������Ҷ������Ӽ仯ѧ������λ����[Co(en)2Cl2]+��Cl-��Ļ�ѧ��Ϊ���Ӽ���H2O�������Ϊ����Լ����Թ��ۼ����Ҷ��������к��зǼ��Թ��ۼ�������ѡ��ABCDE��
��3����NiO����ṹ���Եó�C���ӵ����������
��4�������ӻ�����������������������������������������ĸ����������Ƚ��м��㡣
��1��COԭ�ӵĺ˵����Ϊ27��λ��Ԫ�����ڱ��ĵ�4���ڵ�VIII�壬��̬CO�ĵ����Ų�ʽΪ[Ar]3d74s2���ʴ�Ϊ��VIII��[Ar]3d74s2��
��2���Ҷ�����H2N-CH2-CH2-NH2����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4����ȡsp3�ӻ��������Ҷ�������������������Ϊ��λ���N������һ���Ҷ��������ṩ������λ����һ������λ������2��2+2=6���������Ļ�ѧʽ��֪��[Co(en)2Cl2]Cl��HCl��2H2O�����������Ҷ������Ӽ仯ѧ������λ����[Co(en)2Cl2]+��Cl-��Ļ�ѧ��Ϊ���Ӽ���H2O�������Ϊ����Լ����Թ��ۼ����Ҷ��������к��зǼ��Թ��ۼ�������ѡ��ABCDE���ʴ�Ϊ��sp3��6��ABCDE��
��3����NiO�����������������AΪ��0��0��0����BΪ��1/2��0��0��������ͼ�ɿ���C�����������Ϊ��0��1/2��1/2�����ʴ�Ϊ����0��1/2��1/2����
��4��NiXO������xֵΪ0.88����Ni3+������Ϊx����Ni2+������Ϊ88-x���������ӻ�����������������������������������������ĸ����������ȿ�֪��3��x+��88-x����2=100��2����֮�ã�x=24������Ni2+��Ni3+��������֮�ȣ�88-24����24=8��3���ʴ�Ϊ��8��3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ����Ҫ�Ļ���ԭ�ϣ�����һ�ֿ�������Դ�����п�����Ӧ�õĹ��� ǰ����
ǰ����
��1����֪��CH3OH(g)=HCHO(g)+H2(g) ��H=+84kJmol1,
2H2(g)+O2(g)�T2H2O(g) ��H=484kJmol1
��ҵ�ϳ��Լ״�Ϊԭ����ȡ��ȩ����д��CH3OH(g)��O2(g)��Ӧ����HCHO(g)��H2O(g)���Ȼ�ѧ����ʽ:________________________________________________________________________
��2����һ�ݻ�Ϊ2L���ܱ������ڣ�����0.2molCO��0.4molH2������ӦCO��g��+2H2��g��![]() CH3OH��g���� CO��ƽ��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH��g���� CO��ƽ��ת�������¶ȣ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
��A��B�����Ӧ��ѹǿ��С��ϵ��PA________PB���>��<��=����
������������˵��������Ӧ�ܴﵽ��ѧƽ��״̬����___________(�����)
a.H2������������CH3OH�������ʵ�2�� b.CH3OH������������ٸı�
c.���������ܶȲ��ٸı� d.�����ƽ����Է���������ѹǿ���ٸı�
����P1ѹǿ��T1��Cʱ���÷�Ӧ��ƽ�ⳣ��K=_________(�������)
��T1��C��1L���ܱ������ڷ���������Ӧ�����ijʱ�̸����ʵ����ʵ������£�CO��0.1mol�� H2 ��0.2mol�� CH3OH��0.2mol����ʱv�� ________ v������> �� < �� =����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ����
A. ���³�ѹ�£�124 g P4������P-P����ĿΪ4NA
B. ��״���£�11.2 L�������ϩ������к���ԭ����ĿΪ2NA
C. 1mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA
D. 13g��![]() C��
C��![]() ��ɵ�̼����������������һ��Ϊ6NA
��ɵ�̼����������������һ��Ϊ6NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪X��Y������Ԫ�أ�IΪ�����ܣ���λ��![]() ����������������жϣ��������
����������������жϣ��������
Ԫ�� |
|
|
|
|
X | 496 | 4562 | 6912 | 9543 |
Y | 578 | 1817 | 2745 | 11575 |
A. Ԫ��X�ij������ϼ���![]() ��
��
B. Ԫ��Y�Ǣ�A��Ԫ��
C. X���ʵ��۵����Y���ʵ��۵�
D. ��Ԫ��X���ڵ�3���ڣ����ĵ��ʿ�����ˮ���ҷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵���ͽ��ʹ������
A. ���ڻ�ʱ�����ƻ������е�ȫ����������Ա��ڻ�ʱ�ܶȱ��
B. ±����![]() ��F��I�����ڷ����������Ӽ䷶�»��������۷е�Ҳ������
��F��I�����ڷ����������Ӽ䷶�»��������۷е�Ҳ������
C. ����ʯī�����в����Զ�����Ӳ���ʵ��Ǩ�ƣ�����ʯī�ĵ�����ֻ����ʯīƽ��ķ���
D. �����еġ����������ڵ糡�п��Զ����ƶ������Խ����������õĵ����ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������䣬C�����ʵ�������(C)���¶�(T)��ѹǿ(P)��ϵ��ͼ�����������ϵͼ���ǣ� ��
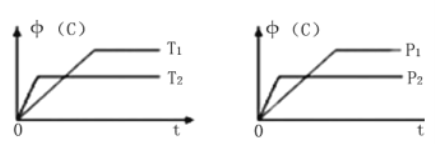
A. 3A(g)+B(s)![]() C(g)+D(g)����H��0
C(g)+D(g)����H��0
B. A(g)+B(s)![]() C(g)+D(g)����H��0
C(g)+D(g)����H��0
C. A(g)+B(s)![]() 2C(g)+D(g)����H��0
2C(g)+D(g)����H��0
D. A(g)+2B(s)![]() C(g)+3D(g)����H��0
C(g)+3D(g)����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��˵��������������ʵ��� ( )
A. ������ʹʯ����Һ���
B. ![]() ʱ.
ʱ.![]() �����pHԼΪ3
�����pHԼΪ3
C. ������м�������������Һ����ҺpH����
D. ��������̼��Ʒ�Ӧ����![]() ����
����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʯī�缫��ⱥ��ʳ��ˮ��ԭ��ʾ��ͼ��ͼ��ʾ������˵����ȷ���� ( )

A. M����
B. ͨ��ʹ�Ȼ��Ʒ�������
C. c�����ռ���������������
D. ���һ��ʱ���������pH����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NaOH��Na2CO3�����Һ�еμ�0.1mol/Lϡ���ᣬCO2���������������������(V)�Ĺ�ϵ��ͼ��ʾ.�����ж���ȷ����(
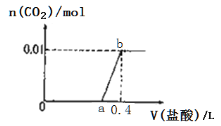
A. ��0~a��Χ�ڣ�ֻ�����кͷ�Ӧ
B. ԭ�����Һ��NaOH��Na2CO3�����ʵ���֮��Ϊ1:2
C. a = 0.3
D. ab�η�����Ӧ�����ӷ���ʽΪ:CO32-+2H+=H2O+CO2��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com