
�Ѻ�ɳ(��Ҫ�ɷ���SiO2)��þ�۰�һ��������(������Լ����15��24)��Ͼ��ȣ�װ���Թ��м��ȣ���Լһ���Ӻ������ҷ�Ӧ������һ�ְ�ɫ���廯�����һ�ֹ軯������ɵĻ������ȴ�����ʢ��ϡ������ձ��У������������ݲ����б�ը����������������һ�����ڿ�������ȼ�Ĺ����̬�⻯�
(1)д����ɳ��þ���ڼ��������·�Ӧ�Ļ�ѧ����ʽ________________���÷�Ӧ��________(����ȡ������ȡ�)��Ӧ��
(2)���ɵĻ�������ϡ�����У�������������(�ѧʽ)________��
(3)д����������ը�����Ļ�ѧ����ʽ______________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ���Ƿ���ij�����ӣ����в���������ȷ���� (����)
A������AgNO3��Һ���а�ɫ�������ɣ�֤����Cl��
B���������ữ��ȡ�ϲ���Һ������Ba(OH)2��Һ��������ɫ������֤����SO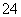
C����ŨNaOH��Һ����ȣ�����ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬֤����NH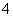
D���������ᣬ�ų���ʹ����ʯ��ˮ����ǵ����壬֤������CO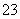
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��黯�����У���ѧ��������ͬ����(˫ѡ)(����)
A��CaCl2��Na2S B��Na2O��Na2O2
C��CO2��CS2 D��HCl��NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ɸ�������ɺ����ʽ��з��ࣺ
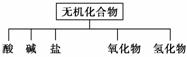
��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢĺ��档
| ���� ��� | �� | �� | �� | ������ | �⻯�� |
| ��ѧ ʽ | ��HCl ��______ | ��______ ��Ba(OH)2 | ��Na2CO3 ��______ | ��CO2 ��Na2O | ��NH3 ��H2O2 |
(1)д����ת��Ϊ�ݵĻ�ѧ����ʽ��__________________________________________
________________________________________________________________________��
(2)д��ʵ�����ɢ��Ʊ�O2�Ļ�ѧ����ʽ��___________________________________
________________________________________________________________________��
(3)ʵ�����Ʊ��߳���________��________��Ӧ�����������ķ�����____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���С�ˮ��֮�硱���ƵĽ��ն�����ʢ��ˮ�����ִ��ڹ��ҵ��ʲ���ݵ�ˮ�������ͳ��Զ����ء�ˮ���ǽϴ���������ʯӢ���壬ʯӢ����Ҫ�ɷ���SiO2�������й�ʯӢ����������ȷ����(����)
A��ʯӢ������ɫ���ľ��壬������װ��Ʒ��Ҳ����������ѧ����
B��ʯӢ����������������
C��ʯӢ���������ȡ���ɰ
D��ʯӢ����������ȡ�ߴ��裬Ҳ�����ƹ��ά
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ȷ����(����)
A��ʯ��Ҥ��������¯�������������Ҫ�ɷֲ���ͬ
B����ʯ��Ĩǽ����ˮ����ǽ�����̵�Ӳ��ԭ����ͬ
C���������մɡ�ˮ���������������������
D���κ�������������������ﷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ش��������⣺
(1)���������������ǽ������ϵ���(����)
A����ǿ��ˮ�� B���ֻ�����
C���������մ� D�����ά
(2)������մ�Ҳ��һ���������ǽ������ϣ��������������������������������������ϵ�(����)
A�����µ��ص� B����ѧ����
C����ѧ���� D�����﹦��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ɫ����ˮ�м���������þ�ۣ��������ˣ���Һ����________ɫ��ԭ���û�ѧ����ʽ��ʾΪ_________________________________________________________��
������Һ�м�����������ˮ����Һ��______ɫ��ԭ�������ӷ���ʽ��ʾΪ_________��
�������Һ�м�����������������Һ����Һ����________���ɣ�ԭ�������ӷ���ʽ��ʾΪ_________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 9.2 g������Ͷ�뵽��������ˮ(2H216O)�У�������������к���
9.2 g������Ͷ�뵽��������ˮ(2H216O)�У�������������к���
 A��0.2 mol���� B��0.4 mol����
A��0.2 mol���� B��0.4 mol����  C��0.2 mol���� D��0.4 mol����
C��0.2 mol���� D��0.4 mol����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com