
 ��
������ AΪԪ��������ԭ�Ӱ뾶��С��Ԫ�أ���A���⣬B���������������������������2����B��̼��CΪ�ؿ��к�������Ԫ�أ���C������A��D��C��F�ֱ�Ϊͬ����Ԫ�أ�����D���ƣ�F����D��E��F���������������Ӧ��ˮ��������֮�䶼�ܷ�����Ӧ������E�������ɴ˷������
��� �⣺��1����������Ԫ�طֱ���H��C��O��Na��Al��S�����Ӳ���Խ��뾶Խ���Ӳ���ͬ�˵����Խ��뾶ԽС������ԭ�Ӱ뾶������Na���ʴ�Ϊ��Na����2��Sλ��Ԫ�����ڱ��е������ڢ�A�壬�ʴ�Ϊ��������A��
��3��������̼�ĵ���ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4����������������������Һ�ķ�Ӧ����ƫ�����ƺ�ˮ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O���ʴ�Ϊ��Al��OH��3+OH-=AlO2-+2H2O��
��5����������������к��еĻ�ѧ��Ϊ���Ӽ��ͷǼ��Թ��ۼ����������ƺ�ˮ֮��ķ�Ӧ����ʽ2Na2O2 +2H2O�T4NaOH+O2�����ڴ˷�Ӧ�У�1mol�������Ʋμӷ�Ӧת�� 1mol���ӣ��ʴ�Ϊ�����Ӽ��ͷǼ��Թ��ۼ���2Na2O2 +2H2O�T4NaOH+O2����1��
���� ���⿼��ԭ�ӽṹ��Ԫ�ص����ʣ�������ѧ���ķ��������Ŀ��飬Ԫ�ص��ƶ��ǽ��Ĺؼ���ע�����Ԫ�ص����ʼ����ʡ�����������ʼ��ɽ����Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
 ��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ��
��ͼ��ʾA��B��C��D��E�������ʵ��ת����ϵ������AΪ����ɫ���壬CΪ�������ʣ�DΪ��õĵ�ζƷ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 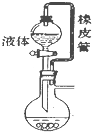 ��Ƥ����ʹҺ��˳������ | B�� | 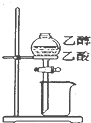 �����Ҵ������� | ||
| C�� | 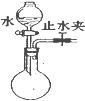 ���װ�������� | D�� | 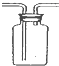 �ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
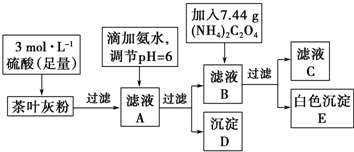
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʳ����ϴ����ϵ�ˮ����CaCO3+2H+�TCa2++CO2��+H2O | |
| B�� | Fe��SCN��3��Һ�е�NaOH��Һ��Fe��SCN��3+3OH-�TFe��OH��3��+3SCN- | |
| C�� | ̼������Һ�Լ��ԣ�CO32-+2H2O?H2CO3+2OH- | |
| D�� | ���ڿ����м���ȼ�գ�4Na+O2$\frac{\underline{\;\;��\;\;}}{\;}$2Na2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com