
ЁОЬтФПЁПгаЛњЮяAЃЈC6H8O4ЃЉЮЊЪГЦЗАќзАжНЕФГЃгУЗРИЏМСЁЃAПЩвдЪЙфхЫЎЭЪЩЋЁЃAФбШмгкЫЎЃЌЕЋдкЫсадЬѕМўЯТПЩЗЂЩњЫЎНтЗДгІЃЌЕУЕНB(C4H4O4)КЭМзДМЁЃЭЈГЃзДПіЯТBЮЊЮоЩЋОЇЬхЃЌФмгыЧтбѕЛЏФЦШмвКЗЂЩњЗДгІЁЃ
ЃЈ1ЃЉAПЩвдЗЂЩњЕФЗДгІга_______________ЃЈбЁЬюађКХЃЉЁЃ
ЂйМгГЩЗДгІ ЂкѕЅЛЏЗДгІ ЂлМгОлЗДгІ ЂмбѕЛЏЗДгІ
ЃЈ2ЃЉBЗжзгЫљКЌЙйФмЭХЕФУћГЦЪЧ______________________________ЁЃ
ЃЈ3ЃЉBЗжзгжаУЛгажЇСДЃЌЦфНсЙЙМђЪНЪЧ________________________ЃЌBЕФОпгаЯрЭЌЙйФмЭХЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЪЧ_________________ЁЃ
ЃЈ4ЃЉгЩBжЦШЁAЕФЛЏбЇЗНГЬЪНЪЧ____________________________________ЁЃ
ЃЈ5ЃЉЬьУХЖЌАБЫсЃЈC4H7NO4ЃЉЪЧзщГЩШЫЬхЕААзжЪЕФАБЛљЫсжЎвЛЃЌПЩгЩBЭЈЙ§вдЯТЗДгІжЦШЁЃК
![]()
ЬьУХЖЌАБЫсЕФНсЙЙМђЪНЪЧ______________________________________ЁЃ
ЁОД№АИЁПЂйЂлЂм ЬМЬМЫЋМќЁЂєШЛљ HOOCCH=CHCOOH CH2=C(COOH)2 HOOCCH=CHCOOH+2CH3OH![]() CH3OOCCH=CHCOOCH3+2H2O
CH3OOCCH=CHCOOCH3+2H2O 
ЁОНтЮіЁП
AЕФЗжзгЪНЮЊC6H8O4ЃЌAЕФВЛБЅКЭЖШЮЊ3ЃЌAПЩвдЪЙфхЫЎЭЪЩЋЃЌAжаКЌЬМЬМЫЋМќЃЛAдкЫсадЬѕМўЯТПЩЗЂЩњЫЎНтЗДгІЕУЕНBЃЈC4H4O4ЃЉКЭCH3OHЃЌBФмгыЧтбѕЛЏФЦШмвКЗДгІЃЌBжаКЌСНИієШЛљКЭЬМЬМЫЋМќЃЌAжаКЌСНИіѕЅЛљЃЛ
ЃЈ1ЃЉAжаКЌгаЬМЬМЫЋМќКЭѕЅЛљЃЌAжаКЌЬМЬМЫЋМќЃЌAФмЗЂЩњМгГЩЗДгІЁЂМгОлЗДгІЁЂбѕЛЏЗДгІЃЌAжаВЛКЌДМєЧЛљКЭєШЛљЃЌAВЛФмЗЂЩњѕЅЛЏЗДгІЃЌД№АИбЁЂйЂлЂмЁЃ
ЃЈ2ЃЉBЗжзгжаЫљКЌЙйФмЭХЮЊЬМЬМЫЋМќКЭєШЛљЁЃ
ЃЈ3ЃЉBЗжзгжаУЛгажЇСДЃЌBЕФНсЙЙМђЪНЮЊHOOCCH=CHCOOHЃЛBЕФОпгаЯрЭЌЙйФмЭХЕФЭЌЗжвьЙЙЬхЕФНсЙЙМђЪНЮЊCH2=C(COOH)2ЁЃ
ЃЈ4ЃЉBгыCH3OHЗЂЩњѕЅЛЏЗДгІЩњГЩAЃЌгЩBжЦШЁAЕФЛЏбЇЗНГЬЪНЮЊHOOCCH=CHCOOH+2CH3OH![]() CH3OOCCH=CHCOOCH3+2H2OЁЃ
CH3OOCCH=CHCOOCH3+2H2OЁЃ
ЃЈ5ЃЉBЕФНсЙЙМђЪНЮЊHOOCCH=CHCOOHЃЌBгыHClЗЂЩњМгГЩЗДгІЩњГЩCЃЌCЕФНсЙЙМђЪНЮЊHOOCCH2CHClCOOHЃЌCгыNH3ЗДгІЩњГЩЬьУХЖЌАБЫсЃЌЬьУХЖЌАБЫсЃЈC4H7NO4ЃЉЪЧзщГЩШЫЬхЕААзжЪЕФАБЛљЫсжЎвЛЃЌдђЬьУХЖЌАБЫсЕФНсЙЙМђЪНЮЊ ЁЃ
ЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГаЫШЄаЁзщЬНОПвдУЂЯѕNa2SO4ЁЄ10H2OКЭCaOЮЊдСЯжЦБИNa2CO3ЁЃ
ЃЈ1ЃЉНЋCaOЫЎЛЏКѓЃЌгыУЂЯѕаЮГЩNa2SO4ЃCa(OH)2ЃH2OШ§дЊЬхЯЕЃЌЗДгІКѓЙ§ТЫЃЌЯђТЫвКжаЭЈШыCO2ЃЌЦкЭћЕУЕНNa2CO3ЁЃШ§дЊЬхЯЕжаЗДгІЕФРызгЗНГЬЪНЮЊЃК SO42Ѓ+ Ca(OH)2(s)+2H2O![]() CaSO4ЁЄ2H2O(s)+2 OHЃ
CaSO4ЁЄ2H2O(s)+2 OHЃ
ИУЗДгІЕФЦНКтГЃЪ§БэДяЪНK=_________________________ЁЃ
ЭљNa2SO4ЃCa(OH)2ЃH2OШ§дЊЬхЯЕжаЬэМгЪЪСПЕФФГжжЫсадЮяжЪЃЌПижЦpH=12.3 [МДc(OHЃ)=0.02mol/L]ЃЌПЩЪЙЗДгІдкГЃЮТЯТШнвзНјааЁЃЗДгІКѓЙ§ТЫЃЌдйЯђТЫвКжаЭЈШыCO2ЃЌНјвЛВНДІРэЕУЕНNa2CO3ЁЃ
ЃЈ2ЃЉдкNa2SO4ЃCa(OH)2ЃH2OШ§дЊЬхЯЕжаВЛжБНгЭЈШыCO2ЃЌЦфРэгЩЪЧ_______________________________________________________________ЁЃ
ЃЈ3ЃЉЬэМгЕФЫсадЮяжЪаыТњзуЕФЬѕМўЃЈаДГіСНЕуЃЉЪЧ_____________ЁЂ______________ЁЃ
ЃЈ4ЃЉгУЦНКтвЦЖЏдРэНтЪЭЬэМгЫсадЮяжЪЕФРэгЩЃК____________________________________ЃЛвдHAБэЪОЫљЬэМгЕФЮяжЪЃЌдђзмЗДгІЕФРызгЗНГЬЪНПЩаДЮЊ_______________________ЁЃ
ЃЈ5ЃЉNa2CO3ШмвКжаДцдкЫЎНтЦНКтЃКCO32ЃЃЋH2O![]() HCO3ЃЃЋOHЃЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ_________ЁЃ
HCO3ЃЃЋOHЃЁЃЯТСаЫЕЗЈДэЮѓЕФЪЧ_________ЁЃ
aЃЎМгЫЎЯЁЪЭЃЌШмвКжаЫљгаРызгЕФХЈЖШЖММѕаЁ
bЃЎЭЈШыCO2ЃЌШмвКpHМѕаЁ
cЃЎМгШыNaOHЙЬЬхЃЌ![]() МѕаЁ
МѕаЁ
dЃЎЯЁЪЭШмвКЃЌЦНКтГЃЪ§діДѓ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПНсОЇСђЫсбЧЬњВПЗжЪЇЫЎЪБЃЌЗжЮіНсЙћШчШдАДFeSO4ЁЄ7H2OЕФжЪСПЗжЪ§МЦЫуЃЌЦфжЕЛсГЌЙ§100ЃЅЁЃЙњМвБъзМЙцЖЈЃЌFeSO4ЁЄ7H2OЕФКЌСПЃКвЛМЖЦЗ99.50ЃЅЁЋ100.5ЃЅЃЛЖўМЖЦЗ99.00ЃЅЁЋ100.5ЃЅЃЛШ§МЖЦЗ98.00ЃЅЁЋ101.0ЃЅЁЃ
ЮЊВтЖЈбљЦЗжаFeSO4ЁЄ7H2OЕФжЪСПЗжЪ§ЃЌПЩВЩгУдкЫсадЬѕМўЯТгыИпУЬЫсМиШмвКНјааЕЮЖЈЁЃ
5Fe2ЃЋЃЋMnO4ЃЃЋ8HЃЋЁњ5Fe3ЃЋЃЋMn2ЃЋЃЋ4H2OЃЛ
2MnO4ЃЃЋ5C2O42ЃЃЋ16HЃЋЁњ2Mn2ЃЋЃЋ10CO2ЁќЃЋ8H2O
ВтЖЈЙ§ГЬЃКДжХфвЛЖЈХЈЖШЕФИпУЬЫсМиШмвК1LЃЌШЛКѓГЦШЁ0.200 g ЙЬЬхNa2C2O4ЃЈЪНСПЮЊ134.0ЃЉЗХШызЖаЮЦПжаЃЌгУеєСѓЫЎШмНтВЂМгЯЁСђЫсЫсЛЏЃЌМгШШжС70ЁцЁЋ80ЁцЁЃ
(1)ШєвЊгУЕЮЖЈЗЈВтЖЈЫљХфЕФИпУЬЫсМиШмвКХЈЖШЃЌЕЮЖЈжеЕуЕФЯжЯѓЪЧ_______________ЁЃ
(2)НЋШмвКМгШШЕФФПЕФЪЧ____ЃЛЗДгІИеПЊЪМЪБЗДгІЫйТЪНЯаЁЃЌЦфКѓвђЗЧЮТЖШвђЫигАЯьЖјдіДѓЃЌИљОнгАЯьЛЏбЇЗДгІЫйТЪЕФЬѕМўЗжЮіЃЌЦфдвђПЩФмЪЧ______________________ЁЃ
(3)ШєЕЮЖЈЪБЗЂЯжЕЮЖЈЙмМтзьВПЗжгаЦјХнЃЌЕЮЖЈНсЪјЦјХнЯћЪЇЃЌдђВтЕУИпУЬЫсМиХЈЖШ_____ЃЈЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЁАЮогАЯьЁБЃЉЁЃ
(4)ЕЮЖЈгУШЅИпУЬЫсМиШмвК29.50mLЃЌдђc(KMnO4)ЃН_____mol/LЃЈБЃСєЫФЮЛгааЇЪ§зжЃЉЁЃ
(5)ГЦШЁЫФЗнFeSO4ЁЄ7H2OЪдбљЃЌжЪСПОљЮЊ0.506gЃЌЃЌгУЩЯЪіИпУЬЫсМиШмвКЕЮЖЈДяЕНжеЕуЃЌМЧТМЕЮЖЈЪ§Он
ЕЮЖЈДЮЪ§ ЪЕбщЪ§Он | 1 | 2 | 3 | 4 |
V(ИпУЬЫсМи)/mL(ГѕЖСЪ§) | 0.10 | 0.20 | 0.00 | 0.20 |
V(ИпУЬЫсМи)/mL(жеЖСЪ§) | 17.76 | 17.88 | 18.16 | 17.90 |
ИУЪдбљжаFeSO4ЁЄ7H2OЕФКЌСПЃЈжЪСПЗжЪ§ЃЉЮЊ_________ЃЈаЁЪ§ЕуКѓБЃСєСНЮЛЃЉЃЌЗћКЯЙњМв______МЖБъзМЁЃ
(6)ШчЪЕМЪзМШЗжЕЮЊ99.80%ЃЌЪЕбщОјЖдЮѓВю=____%ЃЌШчВйзїжаВЂЮоЪдМСЁЂЖСЪ§гыжеЕуХаЖЯЕФЪЇЮѓЃЌдђв§Ц№ЮѓВюЕФПЩФмдвђЪЧЃК__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПдквЛЖЈЮТЖШЯТЃЌНЋЕШСПЕФЦјЬхЗжБ№ЭЈШыЦ№ЪМЬхЛ§ЯрЭЌЕФУмБеШнЦїЂёКЭЂђжаЃЌЪЙЦфЗЂЩњЗДгІЃЌt0ЪБШнЦїЂёжаДяЕНЛЏбЇЦНКтЃЌXЁЂYЁЂZЕФЮяжЪЕФСПЕФБфЛЏШчЭМЫљЪОЁЃдђЯТСагаЙиЭЦЖЯе§ШЗЕФЪЧ
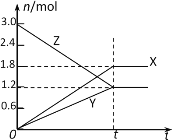
A.ИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊЃК3X+2Y![]() 2Z
2Z
B.ШєСНШнЦїжаОљДяЕНЦНКтЪБЃЌСНШнЦїЕФЬхЛ§V(Ђё)ЃМV(Ђђ)ЃЌдђШнЦїЂђДяЕНЦНКтЫљашЪБМфДѓгкt0
C.ШєСНШнЦїжаОљДяЕНЦНКтЪБЃЌСНШнЦїжаZЕФЮяжЪЕФСПЗжЪ§ЯрЭЌЃЌдђYЮЊЙЬЬЌЛђвКЬЌ
D.ШєДяЦНКтКѓЃЌЖдШнЦїЂђЩ§ИпЮТЖШЪБЦфЬхЛ§діДѓЃЌЫЕУїZЗЂЩњЕФЗДгІЮЊЮќШШЗДгІ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЮТЖШЯТЃЌШѕЫсH2AШмвКжаЃЌДцдкH2AЁЂHAЃКЭA2ЃШ§жжаЮЬЌЕФСЃзгЃЌЦфЮяжЪЕФСПЗжЪ§ІФ[ІФ(X)ЃН![]() ]ЫцШмвКpHБфЛЏЕФЙиЯЕШчЭМЫљЪОЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ
]ЫцШмвКpHБфЛЏЕФЙиЯЕШчЭМЫљЪОЃЌЯТСаЫЕЗЈДэЮѓЕФЪЧ
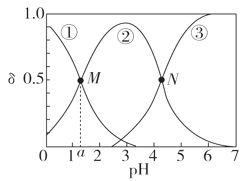
A.pH>4ЕФШмвКжаЃЌІФ(A2Ѓ)ЃН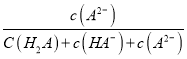 ЃЌ
ЃЌ
B.MЕуЖдгІЕФШмвКжаЫЎЕФЕчРыГЬЖШаЁгкNЕу
C.ШєЭМжаaЮЊ1.2ЃЌдђlg [Ka1(H2A)]ЃНЃ1.2
D.ЧњЯпЂкДњБэЕФСЃзгЪЧHAЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПШчЭМЫљЪОЪЧвЛжжЬьШЛГ§ВнМСЕФНсЙЙЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ
A.ИУГ§ВнМСПЩвдКЭNaHCO3ШмвКЗЂЩњЗДгІ
B.ИУГ§ВнМСЗжзгжаЫљгаЬМдзгвЛЖЈдкЭЌвЛЦНУцФк
C.1 molИУГ§ВнМСзюЖрФмгы6 mol NaOHЗДгІ
D.ИУГ§ВнМСгызуСПЕФH2МгГЩЗДгІЕФВњЮяКЌ7ИіЪжадЬМдзг
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМЪЧЗДгІCO(g)+2H2(g) ЃН CH3OH(g)НјааЙ§ГЬжаЕФФмСПБфЛЏЧњЯпЁЃЯТСаЯрЙиЫЕЗЈе§ШЗЕФЪЧ

A. ИУЗДгІЪЧЮќШШЗДгІ
B. ЪЙгУДпЛЏМСКѓЗДгІШШМѕаЁ
C. ШШЛЏбЇЗНГЬЪНЮЊCO(g)+2H2(g) ЃН CH3OH(g)ЁїHЃН-510 kJЁЄmolЃ1
D. ЧњЯпaБэЪОВЛЪЙгУДпЛЏМСЪБЗДгІЕФФмСПБфЛЏЃЌЧњЯпbБэЪОЪЙгУДпЛЏМСКѓЕФФмСПБфЛЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЖўбѕЛЏТШЃЈClO2ЃЉЪЧвЛжжИпаЇЯћЖОМСЃЌвзШмгкЫЎЃЌЗаЕуЮЊ11.0ЁцЃЌМЋвзБЌеЈЁЃдкИЩдяПеЦјЯЁЪЭЬѕМўЯТЃЌгУИЩдяЕФТШЦјгыЙЬЬхбЧТШЫсФЦжЦБИЖўбѕЛЏТШЃЌзАжУШчЭМЃК
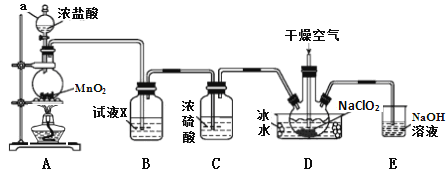
(1)вЧЦїaЕФУћГЦЮЊ_____________ЃЌзАжУAжаЗДгІЕФРызгЗНГЬЪНЮЊ_______________ЁЃ
(2)ЪдМСXЪЧ_______________________ЁЃ
(3)зАжУDжаБљЫЎЕФжївЊзїгУЪЧ___________ЁЃзАжУDФкЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЮЊ_______________ЁЃ
(4)зАжУEжажївЊЗДгІЕФРызгЗНГЬЪНЮЊЃК____________________________ЁЃ
(5)вбжЊNaClO2БЅКЭШмвКдкВЛЭЌЮТЖШЪБЮіГіЕФОЇЬхЧщПіШчЯТБэЁЃ
ЮТЖШ | ЃМ38Ёц | 38ЁцЁЋ60Ёц | ЃО60Ёц |
ЮіГіОЇЬх | NaClO2ЁЄ3H2O | NaClO2 | ЗжНтГЩNaClO3КЭNaCl |
РћгУNaClO2ШмвКжЦЕУNaClO2ОЇЬхЕФВйзїВНжшЃК 55ЁцеєЗЂНсОЇЁЂ_________ЁЂ38ЁЋ60ЁцЕФЮТЫЎЯДЕгЁЂЕЭгк60ЁцИЩдяЁЃ
(6)ЙЄвЕЩЯвВГЃгУвдЯТЗНЗЈжЦБИClO2ЁЃ
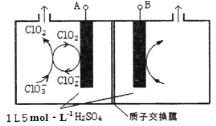
ЂйЫсадЬѕМўЯТЫЋбѕЫЎгыNaClO3ЗДгІЃЌдђЗДгІЕФРызгЗНГЬЪНЮЊ_______________________ЁЃ
ЂкШчЭМЫљЪОЮЊжБНгЕчНтТШЫсФЦЁЂздЖЏДпЛЏбЛЗжЦБИИпДПClO2ЕФЪЕбщЁЃдђвѕМЋЕчМЋЗДгІЪНЮЊ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаНтЪЭЪЕбщЯжЯѓЕФЗНГЬЪНДэЮѓЕФЪЧ
A.ЯђБНЗгШмвКжаж№ЕЮМгШыХЈфхЫЎЃЌЩњГЩАзЩЋГСЕэ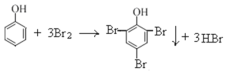
B.ЯђMg(OH)2аќзЧвКжаЕЮМгFeCl3ШмвКЃЌЩњГЩКьКжЩЋГСЕэ3Mg(OH)2 + 2Fe3+ Ёњ 2Fe(OH)3Ё§+ 3Mg2+
C.ЯђЫЎбюЫсЃЈ ![]() ЃЉжаЕЮМгNaHCO3ШмвКЃЌЗХГіЮоЩЋЦјЬхЃК
ЃЉжаЕЮМгNaHCO3ШмвКЃЌЗХГіЮоЩЋЦјЬхЃК![]() + 2HCO3- Ёњ
+ 2HCO3- Ёњ![]() + 2CO2Ёќ + 2H2O
+ 2CO2Ёќ + 2H2O
D.ЯђBa(OH)2жаМгШыH2SO4жСжаадЃКBa2++OH-+SO42-+H+ ЁњBaSO4Ё§+H2O
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com