
ВБ»ТµД»ШКХАыУГ·Ѕ·ЁєЬ¶аЈ¬ПЦУГє¬УРAl2O3Ј¬SiO2єНЙЩБїFeO xFe2O3µДВБ»ТЦЖ±ёAl2(S04)3
xFe2O3µДВБ»ТЦЖ±ёAl2(S04)3 18H2OЈ¬№¤ТХБчіМИзПВЈє
18H2OЈ¬№¤ТХБчіМИзПВЈє
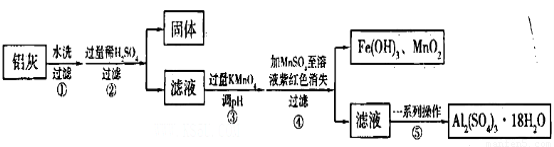
Зл»ШґрПВБРОКМвЈє
ЈЁ1Ј©јУИл№эБїПЎH2SO4ИЬЅвAl2O3µДАлЧУ·ЅіМКЅКЗ______________ЎЈ
ЈЁ2Ј©БчіМЦРјУИЛµДKMnO4ТІїЙУГH2O2ґъМжЈ¬ИфУГH2O2·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄ_______________ЎЈ
ЈЁ3Ј©ТСЦЄЈєЕЁ¶ИѕщОЄO.1mol/LµДЅрКфСфАлЧУЈ¬ЙъіЙЗвСх»ЇОпіБµнµДpHИзПВ±нЈє
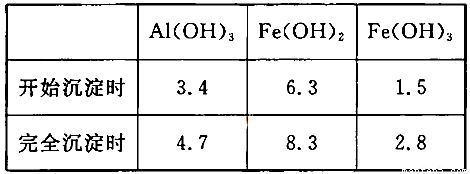
ІЅЦиўЫµДДїµДКЗ__________________________________________________________Ј»ИфФЪёГЕЁ¶ИПВіэИҐМъµД»ЇєПОпЈ¬µчЅЪpHµДЧоґу·¶О§КЗ___________ЎЈ
ЈЁ4Ј©ТСЦЄ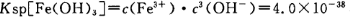 µ±pH=2К±Ј¬Fe3Ј«їЄКјіБµнµДЕЁ¶ИОЄ_______________ЎЈ
µ±pH=2К±Ј¬Fe3Ј«їЄКјіБµнµДЕЁ¶ИОЄ_______________ЎЈ
ЈЁ5Ј©ІЩЧчўЬ·ўЙъ·ґУ¦µДАлЧУ·ЅіМКЅОЄ__________________________________________Ј»ОЄБЛСйЦ¤ёГІЩЧчЛщµГ№ММеЦРИ·Кµє¬УРMnO2Ј¬їЙСЎУГµДКФјБКЗ_________»т_________ЎЈ
ЈЁ6Ј©ІЩЧчўЭЎ°Т»ПµБРІЩЧч"Ј¬ПВБРТЗЖчЦРІ»РиУГµДКЗ___________ЈЁМоРтєЕЈ©ЎЈ
AЈ®Хф·ўГу? BЈ®ЫбЫц? CЈ®ІЈБ§°ф? DЈ®ѕЖѕ«µЖ?? ? EЈ®В©¶·
ЈЁ1Ј©6H+ + Al2O3=2Al3+ + 3H2O ЈЁ2Ј©H2O2 + 2FeSO4 + H2SO4 = Fe2(SO4)3 + 2H2O ЎЈЈЁ3Ј©Ѕ«¶юјЫМъАлЧУСх»ЇИэјЫМъАлЧУЈ¬ІўНЁ№эµчЅЪPHЦµ°СИэјЫМъАлЧУЧЄ»ЇОЄЗвСх»ЇМъіБµніэИҐЎЈ2.8µЅ3.4ЎЈЈЁ4Ј©4*10-2mol/LЎЈЈЁ5Ј©3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O Ј»ЕЁСОЛбЈ»№эСх»ЇЗвИЬТєЎЈЈЁ6Ј© B
ЎѕЅвОцЎї
КФМв·ЦОцЈєЈЁ1Ј©јУИл№эБїПЎH2SO4ИЬЅвAl2O3µДАлЧУ·ЅіМКЅКЗ6H+ + Al2O3=2Al3+ + 3H2O ЎЈЈЁ2Ј©БчіМЦРјУИЛµДKMnO4ТІїЙУГH2O2ґъМжЈ¬H2O2УРЗїСх»ЇРФ°С¶юјЫМъАлЧУСх»ЇОЄИэјЫМъАлЧУЈ¬·ўЙъ·ґУ¦µД»ЇС§·ЅіМКЅОЄH2O2 + 2FeSO4 + H2SO4 = Fe2(SO4)3 + 2H2O ЎЈЈЁ3Ј©ёщѕЭБчіМНјїЙЦЄКЗЅ«¶юјЫМъАлЧУСх»ЇИэјЫМъАлЧУЈ¬ІўНЁ№эµчЅЪPHЦµ°СИэјЫМъАлЧУЧЄ»ЇОЄЗвСх»ЇМъіБµніэИҐЈ¬И·±ЈВБАлЧУІ»ТЄіБµніцАґЈ¬№КµчЅЪpH·¶О§ОЄЈє2.8µЅ3.4ЎЈЈЁ4Ј©ТСЦЄ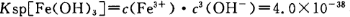 µ±pH=2К±Ј¬јґc(H+)=0.01Mmol/LЈ¬c(OH-)=10-12Mmol/LЈ¬ґъИл
µ±pH=2К±Ј¬јґc(H+)=0.01Mmol/LЈ¬c(OH-)=10-12Mmol/LЈ¬ґъИл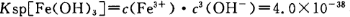 №«КЅЈ¬їЙµГFe3Ј«їЄКјіБµнµДЕЁ¶ИОЄ4*10-2mol/LЎЈЈЁ5Ј©ёщѕЭІЩЧчЦРіцПЦµДПЦПуЈ¬ЧПЙ«ПыК§Ј¬ЛµГчёЯГМЛбёщАлЧУІОјУБЛ·ґУ¦Ј¬ўЬ·ўЙъ·ґУ¦µДАлЧУ·ЅіМКЅОЄ3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O Ј»ОЄБЛСйЦ¤ёГІЩЧчЛщµГ№ММеЦРИ·Кµє¬УРMnO2Ј¬їЙСЎУГµДКФјБКЗ_ЕЁСОЛбІўјУИИ»т№эСх»ЇЗвИЬТєУРЖшЕЭІъЙъјґїЙЛµГчЎЈЈЁ6Ј©ІЩЧчўЭЎ°Т»ПµБРІЩЧч"ЦРУРЈ¬Хф·ўЕЁЛхЈ¬ЅµОВЅбѕ§Ј¬№эВЛЈ¬І»РиУГµДКЗ BЈ®ЫбЫцЎЈ
№«КЅЈ¬їЙµГFe3Ј«їЄКјіБµнµДЕЁ¶ИОЄ4*10-2mol/LЎЈЈЁ5Ј©ёщѕЭІЩЧчЦРіцПЦµДПЦПуЈ¬ЧПЙ«ПыК§Ј¬ЛµГчёЯГМЛбёщАлЧУІОјУБЛ·ґУ¦Ј¬ўЬ·ўЙъ·ґУ¦µДАлЧУ·ЅіМКЅОЄ3Mn2+ + 2MnO4- + 4OH- =5MnO2 + 2H2O Ј»ОЄБЛСйЦ¤ёГІЩЧчЛщµГ№ММеЦРИ·Кµє¬УРMnO2Ј¬їЙСЎУГµДКФјБКЗ_ЕЁСОЛбІўјУИИ»т№эСх»ЇЗвИЬТєУРЖшЕЭІъЙъјґїЙЛµГчЎЈЈЁ6Ј©ІЩЧчўЭЎ°Т»ПµБРІЩЧч"ЦРУРЈ¬Хф·ўЕЁЛхЈ¬ЅµОВЅбѕ§Ј¬№эВЛЈ¬І»РиУГµДКЗ BЈ®ЫбЫцЎЈ
їјµгЈє±ѕМвїјІйАлЧУ·ЅіМКЅµДКйРґЈ¬КµСйФАнµД·ЦОцєНіБµнИЬЅвЖЅєвПа№ШјЖЛгЎЈ


 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ё
| Дкј¶ | ёЯЦРїОіМ | Дкј¶ | іхЦРїОіМ |
| ёЯТ» | ёЯТ»Гв·СїОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СїОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СїОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СїОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СїОіМНЖјцЈЎ | іхИэ | іхИэГв·СїОіМНЖјцЈЎ |
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
| 1 |
| 2 |
| ОВ¶ИЈЁЎжЈ© | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| НЖЅѕщИЬЅвЛЩВК ЈЁЎБ10-3mol?L-1?min-1Ј© |
7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈєС§П°ёЯКЦ±ШРЮ¶ю»ЇС§ВіїЖ°ж ВіїЖ°ж МвРНЈє022
ГєКЗУЙ________єН________ЧйіЙµДёґФУ»мєПОпЈ®Ц±ЅУИјЙХїЙТФІъЙъґуБїµДОЫИѕОпЈ¬ИзМјµДСх»ЇОпЎўµЄµДСх»ЇОпЎўБтµДСх»ЇОпЎўСМіѕМјБЈЈ¬»№УР»У·ўіцАґµДМјЗв»ЇєПОпµИЈ¬ЖдЦРДЬ№»К№УкЛ®Лб»ЇµДУР¶ѕЖшМеКЗ________єН________Ј®¶ФГєЧЫєПАыУГїЙТФ»сµГЅаѕ»µДИјБПєН¶аЦЦ»Ї№¤ІъЖ·Ј¬ГєµДЧЫєПАыУГ·Ѕ·ЁєЬ¶аЈ¬ЖдЦРИзНјЛщКѕЈ¬Ѕ«ГєёфѕшїХЖшјУЗїИИК№Ц®·ЦЅвµД·Ѕ·ЁЅР________Ј¬ёГ№эіМїЙТФІъЙъТєМеB________Ј¬»№ЙъіЙїЙИјРФµДЖшМеЈ¬ХвР©їЙИјРФµДЖшМеУР________(ЧоЙЩРґіц3ЦЦ)Ј»ґУBЦРДЬ№»·ЦАлМбИЎіцТ»ЦЦЖ¬Чґѕ§МеЎЄЎЄЭБЈ¬ЖдЅб№№јтКЅКЗЈє![]() Ј¬Жд·ЦЧУКЅКЗ________Ј®
Ј¬Жд·ЦЧУКЅКЗ________Ј®

Ійїґґр°ёєНЅвОц>>
їЖДїЈєёЯЦР»ЇС§ АґФґЈє МвРНЈє
Ійїґґр°ёєНЅвОц>>
№ъјКѧУУЕСЎ - Б·П°ІбБР±н - КФМвБР±н
єю±±КЎ»ҐБЄНшОҐ·ЁєНІ»БјРЕПўѕЩ±ЁЖЅМЁ | НшЙПУРє¦РЕПўѕЩ±ЁЧЁЗш | µзРЕХ©ЖѕЩ±ЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРє¦РЕПўѕЩ±ЁЧЁЗш | ЙжЖуЗЦИЁѕЩ±ЁЧЁЗш
ОҐ·ЁєНІ»БјРЕПўѕЩ±Ёµз»°Јє027-86699610 ѕЩ±ЁУКПдЈє58377363@163.com