
������ӵĽṹ��ʽ��ͼ��ʾ: ��������ӵ�һ��ͬ���칹��Լ�����������(F)���л�����ԭ��,���ںϳ�ҩ����м��塣ijУ��ȤС���������������ѧ��ѧ�ļ��л���ϳ�F,�䷽������,����A�IJ�����־��һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ��
��������ӵ�һ��ͬ���칹��Լ�����������(F)���л�����ԭ��,���ںϳ�ҩ����м��塣ijУ��ȤС���������������ѧ��ѧ�ļ��л���ϳ�F,�䷽������,����A�IJ�����־��һ������ʯ�ͻ�ѧ��ҵ�ķ�չˮƽ��

��1��д��������ӵķ���ʽ __________________��
д��C���ʵ����� _____________________��
��2��ָ����ѧ��Ӧ����:��Ӧ��________����Ӧ��_________��
��3���������ʲ����붡����ӷ�����Ӧ���� __________________��
a.NaOH��Һ b.NaHCO3��Һ
c.Br2�����Ȼ�̼��Һ d.�Ҵ�
��4��д����Ӧ�۵Ļ�ѧ����ʽ: ____________________________��
��5��д�����������ٺ��б���;���ܹ�����������Ӧ��D��ͬ���칹��Ľṹ��ʽ�� _____________________________________��
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ��ѧ�ڵ��Ĵ��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
þȼ�ϵ�ؾ��б������ߡ�ʹ�ð�ȫ���㡢ԭ������Դ�ḻ���ɱ��͵��ص㡣һ���о���þȼ�ϵ�ؿɷ�Ϊþ����ȼ�ϵ�ء�þ��ˮȼ�ϵ�ء�þ��������ȼ�ϵ�غ�þ��������ȼ�ϵ�ء����У�þ��������ȼ�ϵ�صĹ���ԭ����ͼ��ʾ�������й�˵������ȷ����( )
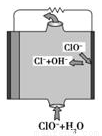
A��þȼ�ϵ����þ��Ϊ����������������Ӧ
B��þ��������ȼ�ϵ�ص��ܷ�ӦʽΪMg��ClO����H2O===Mg(OH)2��Cl��
C��þ��������ȼ�ϵ�أ����Ե������������ӦʽΪH2O2��2H����2e��===2H2O
D��þ��������ȼ�Ϸŵ������OH����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
��һ������:NaOH��H2O��MgBr2 ��CO2 ��Na2O2��H2O2 ��N2��NH4Cl
��1�� ���ӻ�������
���ۻ�������
��2���õ���ʽ��ʾH2O��MgBr2���γɹ���
H2O
MgBr2
��3�� H2O�� ����ϣ�MgBr2�� ����ϡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
ͬ����Ԫ�أ���ԭ�ӽṹ��֮ͬ���� �� ��
A. ���������� B. �˵����
C. ���Ӳ��� D. ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ��һ��ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
������ͨ�����У����������������˹���������������Щ���������ʣ� ��
�ٻ���ɫ ���ܶȱȿ����� ���ж� ����Һ�� ������ˮ
A���٢ڢ� B���ڢ� C���ۢ� D���ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ������
��1����֪2 mol����ȼ������Һ̬ˮʱ�ų�572 kJ��������Ӧ����ʽ��
2H2(g)+O2(g)==2H2O(l)
(l)��ش��������⣺
�ٸ÷�Ӧ�������������ܺ�________(����ڡ�����С�ڡ����ڡ�)��Ӧ�������ܺ͡�
����2 mol������ȫȼ������ˮ��������ų�������________(�����������������)572 kJ��
���뻯ʯȼ����ȣ���������Դ�кܶ��ŵ㣬��˵������һ��______________________��
��2��FeS2���ղ�����SO2�����������ᡣ��֪25 �桢101 kPaʱ��
2SO2(g)��O2(g)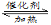 2SO3(g) ��H1����197 kJ��mol��1��
2SO3(g) ��H1����197 kJ��mol��1��
H2O(g)===H2O(l) ��H2����44 kJ��mol��1��
2SO2(g)��O2(g)��2H2O(g)===2H2SO4(l)��H3����545 kJ��mol��1
��SO3(g)��H2O(l)��Ӧ����H2SO4(l)���Ȼ�ѧ����ʽ��_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ȩ����Ũ����������,��ṹ��ʽ��ͼ��ʾ����������ȩ����������������� ( )
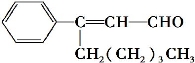
A���ڼ��Ⱥʹ���������,�ܱ�������ԭ
B���ܱ����Ը��������Һ����
C����һ�������������巢��ȡ����Ӧ
D�������������ᷢ���ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������ʡ�߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ��Ƽ�����ᡢ����������ȹ�ϵ���У������й�˵������ȷ���ǣ� ��
A����ϩ�IJ����Ǻ���һ������ʯ�ͻ�����չˮƽ�ı�־
B���Ҵ�������һ�����ȼ�ϣ����Լ����ж��к�����Ի�������Ⱦ
C���ᳫ���ǹ���ʱ�������ϴ�����Ϊ�˷�ֹ��ɫ��Ⱦ
D��35%--40%��ȩ��Һ�׳�Ϊ�������֣����кܺõķ���ɱ��Ч�������������ݺ���Ʒ������Ч��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ���½�ũҵ��ѧ���и߶���ѧ�����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���и������ӣ���ָ���Ļ������ܹ������������( )
ѡ�� | ָ������ | �ܹ���������� |
A | �μӷ�̪�Ժ�ɫ����Һ | K����S2����SO32-��NO3- |
B | ��HCO3-���ڵ���ɫ����Һ | Na����K����Cl����Al3�� |
C | ˮ�����c(H��)��10��12 mol��L��1����Һ�� | Cl����CO32-��NO3-��NH4+ |
D | ʹʯ�������Һ�� | Fe2����MnO4-��NO3-��SO42- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com