
 2Fe2O3+8SO2
2Fe2O3+8SO2 2SO3 �ʴ�Ϊ��2SO2+O2
2SO3 �ʴ�Ϊ��2SO2+O2 2SO3��
2SO3��| �������/g | 10.00 | 20.00 | 30.00 | 40.00 |
| ��NH4HSO4����NH4��2SO4/mol | X��Y | 2X��2Y | 3X��3Y | 4X��4Y |
| ����NH3/mol | X+2Y | X+2Y | 0.04 | 0 |
| ����NaOH/mol | 2X+2Y | 3X+2Y | 3X+0.04 | 3X+0.04 |
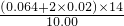 ��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��
��100%=14.56%������15.00 g�����NaOH��Һ��Ӧ������NH3�����������ۿ�֪��NaOH��Һ�й���0.232molNaOH�����������Ϊ15.00gʱ��0.096mol NH4HSO4��0.03mol ��NH4��2SO4������NH4+��H+ 0.252mol����NaOH���㣬��ʱ����n��NH3��=��0.232-0.096��mol=0.136mol��NH3������=0.136mol��17g/mol=2.31g��

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡȪ���е»���2010�������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�022
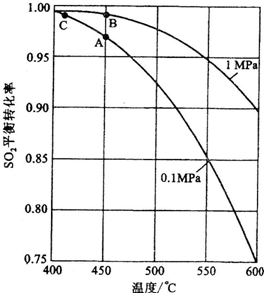
�Ի�����Ϊԭ����������Ĺ�������ͼ���£�
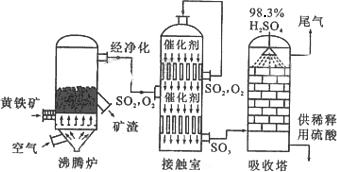
(1)��д������¯�л�����ȼ�յĻ�ѧ����ʽ��________________��
(2)�Ӵ�����2SO2(g)
��O2(g)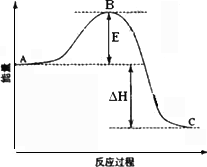
�����ݹ�������ͼ�ж�����˵����ȷ����(ѡ�������ĸ)________��
a��Ϊʹ��������ȼ�գ��轫�����
b���������������SO2��ת����
c��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
d������¯�ų��Ŀ����ɹ�����
�ڷ�Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK��________�������¶ȣ�Kֵ________(���������С�����䡱)��ͼ�С�H��________KJ��mol��1��
��ͼ��C���ʾ________��E�Ĵ�С�Ը÷�Ӧ�ķ�Ӧ��________(��С����ޡ�)Ӱ�죮�÷�Ӧͨ����V2O5����������V2O5��ʹͼ��B��________(����ߡ����͡�)��
�������Ӧ����v(SO2)Ϊ0.05 mol��L��1��min��1����v(O2)��________mol��L��1��min��1��
����֪�������ȼ����Ϊ��296 KJ��mol��1��������S(s)����3 mol��SO3(g)�ġ�H��________��
(3)�������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�����õ��ϸ�Ũ�ȵ�SO2����Σ�SO2�ȿ���Ϊ���������ԭ��ѭ�������ã�Ҳ�����ڹ�ҵ������������ճ�ʪ�����е�Br2��SO2����Br2�����ӷ���ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
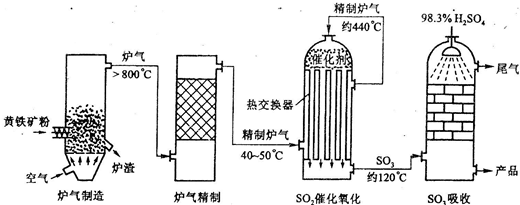
��1��FeS2��s��+11/4O2��g��====1/2Fe2O3��s��+2SO2��g������H=��835 kJ��mol��1
��2��SO2��g��+1/2O2��g��====SO3��g������H=��98.3 kJ��mol��1
��3��SO3��g��+H2O��l��====H2SO4��l������H=��130.3 kJ��mol��1
���㽫1.0 mol FeS2�е���ȫ��ת��ΪH2SO4�������Ͽɲ�������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
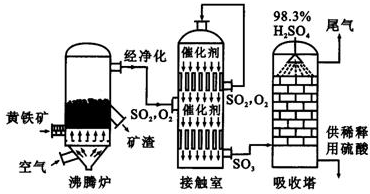
��ҵ���Ի�����Ϊԭ������������Ҫ��Ϊ�����ν��У����ڷ���¯�����ջ�����SO2�Ĵ���������SO3�����ա���ش����м����й����Ṥҵ�еļ������⡣
��1��������������ϵķ��������̷�Ϊԭ�ϣ������������ա���Ӧ�Ļ�ѧ����ʽΪ��2FeSO4��7H2O ![]() Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )
Fe2O3 + SO2��+ SO3��+ 14H2O����������������ˮ����ͬʱ������õ����ᡣ����ͼ��ʾװ��ģ�����̷��������ʵ�飬���������ɵ�����Ͷ���������װ������ȥ��������bΪ������Թܡ����й��ڸ÷�Ӧ˵����ȷ����( )

A��������Ӧ�����������ͨ��BaCl2��Һ�У������ij���ΪBaSO3��BaSO4
B��b�в�������ɫʯ����Һ���ɼ��������H+��SO42��
C��Ϊ���鷴Ӧ����һ��������Թ�c��Ӧ������Լ�ΪNaOH��Һ
D��b�����õ����������������Ϊ29.5%
(2)�ӷ���¯�г�����¯�����뾭������ϴ�ӡ���������Ӵ��ң�����ҪĿ����__________��
(3)�Ӵ������Ƚ�������ʵ�����Ƚ�����װ�á���ѧʵ����Ҳ���������Ƚ�����ʵ��ij��ʵ��Ŀ�ģ�������Һ�Ƚ���ʱͨ��ʹ�õ�������______________��
(4)�Ӵ�������Ҫ��Ӧ��SO2�Ĵ�����������������Ĺ����У�����ý(V2O5)�����ܼӿ���������������ٶȣ����˾������������⣬������Ϊ��Ӧ�����л�������һ�������м���(��ͼ)��c���Ļ�ѧ����ʽ�ɱ�ʾΪ_______________________��

(5) ��ҵ����������Ϊԭ����������������β�����˺���N2��O2�⣬������SO2������SO3��������Ϊ�˱���������ͬʱ������Ṥҵ���ۺϾ���Ч�棬Ӧ�����ܽ�β���е�SO2ת��Ϊ���õĸ���Ʒ����β��ͨ���ĩ״��̼��ƻ���ʯ�ҵ�����Һ�У�����һϵ�д�����õ�һ����Է�������Ϊ172�Ļ���ԭ��J����д��J�Ļ�ѧʽ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com