


 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д� �ݾ�ѵ������ϵ�д�
�ݾ�ѵ������ϵ�д� С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
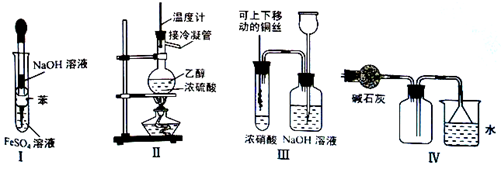
| A�����â���ȡFe(OH)2 |
| B�����â�װ����ȡC2H4 |
| C�����â�װ�ý���ͭ��Ũ���ᷴӦ��ʵ�� |
| D�����â�װ���ռ�NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������е���Ԫ��ʱ���������������������Һ��ϼ��Ⱥ���������ữ |
| B��Ϊ�ӿ�����ٶȣ����ò���������������е�ʳ��ˮ |
| C��ΪѸ�ٳ�ȥ���������е��������ᣬ�ɼ�������NaOH��Һ������ |
| D��Ϊʹ��ȡH2�����ʼӿ죬����ϡH2SO4�м�����CuSO4��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
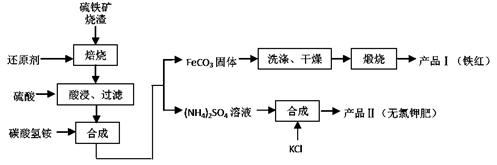
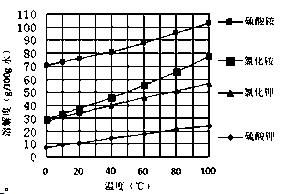
 2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǣ���
2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǣ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺
OC�DCOOH���ɼ�дΪH2C2O4)�׳Ʋ��ᣬ������ˮ�����ڶ�Ԫ��ǿ��(Ϊ�������)��������ǿ��̼�ᣬ���۵�Ϊ101.5�棬��157��������ijУ�о���ѧϰС��Ϊ̽������IJ��ֻ�ѧ���ʣ�����������ʵ�飺 HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______
HCO3��Һ���Թ��м��������Ҷ�����Һ���۲쵽����ɫ���ݲ������÷�Ӧ�����ӷ���ʽΪ_______ __________________________________��
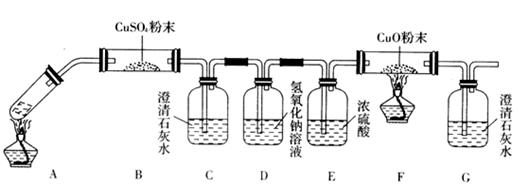
__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
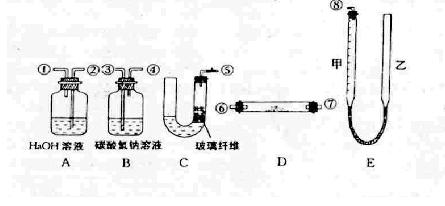
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʵ����ϵ��������¶Ⱥ�ʱ�䶼������ʵ��ı��� |
| B������ʵ���ж��������ÿհ����飬ȷ����һ���� |
| C�����ݲ���Ӧ����ȷ�������Ҫ��β�����ƽ��ֵ |
| D��̽��ʵ������У�ʵ��������������ļ���һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
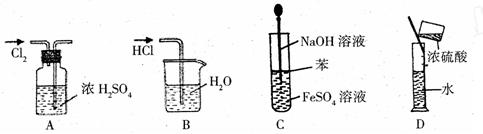
| A������Cl2 | B������HCl |
| C����ȡFe(OH)2���� | D��ϡ��ŨH2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ��Һ��ȥ
��Һ��ȥ ���Ȼ������ɫ����
���Ȼ������ɫ����| A���٢ڢۢ� | B���ڢܢݢ� | C���٢ۢݢ� | D���٢ڢۢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com