
| A�� | ��Һ��һ����K+��Br-��CO32-��AlO2- | |
| B�� | ��Һ��һ��û��Mg2+��Cu2+��Cl-��NH4+ | |
| C�� | ����ȷ����Һ���Ƿ���K+��SO42-��Cl- | |
| D�� | ����3����Һ�еμ�BaCl2����ȷ���Ƿ���SO42- |
���� �����Թ�ȡ������Һ����μ���ϡ��������������Һ�Ȼ��Ǻ��ֱ���壬����ɫ����ų�����֪��ɫ����Ϊ������̼��һ����CO32-����Һ�Ȼ��Ǻ��ֱ�����֪һ����AlO2-�������ӹ����֪��һ������H+��Mg2+��Cu2+��
����һ����Һ����μ���NaOH��Һ����������Һ�Ȼ��Ǻ��ֱ���壬���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�δ������������NH4+��
������һ����Һ�м������Ƶ���ˮ��CCl4�����ã��²���Һ�ԳȺ�ɫ����һ����Br-����ϵ���غ㼰���ӹ�����
��� �⣺�����Թ�ȡ������Һ����μ���ϡ��������������Һ�Ȼ��Ǻ��ֱ���壬����ɫ����ų�����֪��ɫ����Ϊ������̼��һ����CO32-����Һ�Ȼ��Ǻ��ֱ�����֪һ����AlO2-�������ӹ����֪��һ������H+��Mg2+��Cu2+��
����һ����Һ����μ���NaOH��Һ����������Һ�Ȼ��Ǻ��ֱ���壬���ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�δ������������NH4+��
������һ����Һ�м������Ƶ���ˮ��CCl4�����ã��²���Һ�ԳȺ�ɫ����һ����Br-���ɵ���غ��֪һ������������ΪK+������ȷ���Ƿ�Cl-��SO42-��
A��������������֪��Һ��һ������K+��Br-��CO32-��AlO2-����A��ȷ��
B������ȷ���Ƿ�Cl-����B����
C����Һ��һ����K+����C����
D�����ڵ�3����Һ�����Ѿ�����HCl��Ӧ����CO32-�����ٵμ�BaCl2�����ɵij�����ΪBaSO4������ȷ��SO42-�Ĵ��ڣ���D����
��ѡA��
���� ���⿼�����ӵĹ��棬Ϊ��Ƶ���㣬��Ŀ�Ѷ��еȣ�������Ϣ���ϴ���Ҫѧ�������Ķ���Ϣ���������ӵķ�Ӧ����������۵Ĺ�ϵΪ���Ĺؼ������ط������ƶ������Ŀ��飮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʺ���������һ����������ȫȼ�ն�ֻ����CO2��ˮ | |
| B�� | 75%��������������Ҵ���Һ������ҽ������ | |
| C�� | ʯ�͵ķ����ú�ĸ�������������仯 | |
| D�� | ��ϩ�ͱ�����ʹ��ˮ��ɫ����ɫ��ԭ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 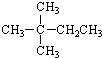 | B�� |  | C�� | CH3CH2CH2CH2CH3 | D�� | CH3CH2CH2CH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
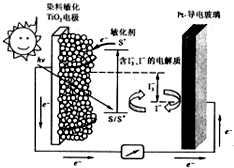 ��ͼ��һ��Ⱦ������̫���ܵ�ص�ʾ��ͼ����ص�һ���㼫���л�����ȼ�ϣ�S��Ϳ����TiO2����������Ƴɣ���һ�缫�ɵ��粣���Ʋ����ɣ�����з����ķ�ӦΪ��
��ͼ��һ��Ⱦ������̫���ܵ�ص�ʾ��ͼ����ص�һ���㼫���л�����ȼ�ϣ�S��Ϳ����TiO2����������Ƴɣ���һ�缫�ɵ��粣���Ʋ����ɣ�����з����ķ�ӦΪ��| A�� | ��ع���ʱ���ǽ�̫����ת��Ϊ���� | |
| B�� | ����жƲ����粣��Ϊ���� | |
| C�� | ��ع���ʱ��I-�����ڶƲ����粣���缫�Ϸŵ� | |
| D�� | ��صĵ������Һ��I-��I3-��Ũ�Ȳ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Va��Vbʱ��c��CH3COOH��+c��CH3COO-����c��K+�� | |
| B�� | Va=Vbʱ��c��CH3COOH��+c��H+����c��OH-�� | |
| C�� | Va��Vbʱ��c��CH3COO-����c��K+����c��OH-����c��H+�� | |
| D�� | Va��Vb�����ʱ��c��K+��+c��H+��=c��OH-��+c��CH3COO-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C��Na+����C��HCO3-����C��CO32-����C��H+����C��OH-�� | B�� | C��Na+����C[H+]=C[HCO3-]+C[CO32-]+C[OH-] | ||
| C�� | C[Na+]+C[H+]=C[HCO3-]+2C[CO32-]+C[OH-] | D�� | C[Na+]=C[HCO3-]+C[CO32-] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | .�٢ڢ� | B�� | �٢ڢ� | C�� | �٢ܢݢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v ��NO��=0.0010 mol•L-1•s-1 | B�� | v ��O2��=0.0010 mol•L-1•s-1 | ||
| C�� | v ��NH3��=0.010 mol•L-1•s-1 | D�� | v ��H2O��=0.045 mol•L-1•s-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com