



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B����NaOH��Һ�����ȣ�������ɫʯ���Լ� |
| C����NaOH��Һ�����ȣ������̪�Լ� |
| D����NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������ϳɰ��� |
| B������������ϳ������ |
| C�����������Ʊ����� |
| D���ӿ����з�������� |
�鿴�𰸺ͽ���>>
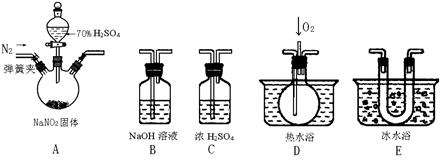
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��hb>hc>ha>hd | B��hc>ha>hb>hd | C��hd>ha>hc>hb | D��hb>ha>hc>hd |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
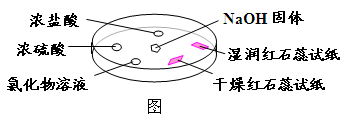
| ѡ�� | ʵ������ | ���� |
| A | Ũ���ḽ���������� | NH3��Ũ���ᷴӦ������NH4Cl���� |
| B | Ũ���ḽ������������ | NH3��Ũ���������Ӧ |
| C | �Ȼ�����Һ����� | ����Һһ����AlCl3��Һ |
| D | �����ʯ����ֽ����ɫ��ʪ���ɫʯ����ֽ���� | NH3��һ�ֿ����Լ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ҵ�ϳɰ��Ĺ����ǹ̵��Ĺ��� |
| B����Ȼ���У����Ƕ������ر��ǵ����ʸ��ܺ�IJ��� |
| C��Ϊ��ֹ��ʳ����ͷ��ˮ����ʳƷ���ã����õ����������� |
| D���������������죬N2��O2�ᷢ����Ӧ������ת��Ϊ�����α�ֲ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A��Ũ�������������ֽ⣬���뱣������ɫƿ�� |
| B��ŨHNO3����ǿ����������ֻ��ʹʪ����ɫ��ʯ����ֽ�Ժ�ɫ������ɫ |
| C��ϡHNO3�ͻ��ý�����Ӧʱ��Ҫ�õ����� |
| D�������£���ŨHNO3��Ͷ��FeƬ������������ĺ���ɫ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com