
���ú�ˮ������ȡ���þ����ȡ�������¡�
(1)��ȡ��Ĺ����У���������Br����Br2ת����Ŀ����________���������з�����Ӧ�����ӷ���ʽ��__________����ƽ���ƶ�ԭ������ͨ��������ҪĿ����_______��
(2)��MgCl2��Һ�еõ�MgCl2��6H2O�������Ҫ������________________�����ˡ�ϴ�ӡ����
(3)�����������̣�����10 m3��ˮ�е���Ԫ��ת��Ϊ��ҵ�壬������Ҫ��״����Cl2�����Ϊ________L(����Cl2���ܽ�)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�Q��W��X��Y��Z�����ڱ�ǰ36��Ԫ���е����ֳ���Ԫ�أ���ԭ��������������Wԭ������������������������֮��Ϊ3��4��Q��Y���������ǵ����������Ҫ���ʣ�X�ǵؿ��к�����ߵĽ���Ԫ�أ�Z���γɺ�ɫ����ש��ɫ����Z2O�ͺ�ɫ��ZO�������������������л�ѧ����ش� ��1��Q���ʵĵ���ʽΪ_______��
��1��Q���ʵĵ���ʽΪ_______�� W��X��Y�����Ӱ뾶�ɴ�С��˳��Ϊ____�������ӷ��Żش𣩡�
W��X��Y�����Ӱ뾶�ɴ�С��˳��Ϊ____�������ӷ��Żش𣩡� ��2��X��Y��ɵĻ��������ˮ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
��2��X��Y��ɵĻ��������ˮ�з�Ӧ�Ļ�ѧ����ʽ��______________________________��
��3��Na2Y��Һ������Ũ���ɴ�С��˳��Ϊ____________________________________________�� ��4��ZO�ڸ����±�Q�ļ���̬�⻯�ﻹԭΪZ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________��
��4��ZO�ڸ����±�Q�ļ���̬�⻯�ﻹԭΪZ���ʣ�д����Ӧ�Ļ�ѧ����ʽ____________��
��5��ZCl2��Һ�л���FeCl3����ʱ���ɼ���_____________�����Լ�������pH��_________���ٹ��ˡ���֪��Fe(OH)3��Ksp��10-35����ѧ����Ϊ��������Һ�е�����Ũ��С��1��10-5mol/Lʱ�������ʹ���ȫ��
��6����ҵ�Ͽ��ø���������Z2Y + O2��2Z + YO2��ұ������Z������1molZʱת��____mol���ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
2013��10��������̨��������ܵ��ش���ʧ���м������Ľ����ɹ�����ҩƷ���������ֺ���Ҫ�����������������������и�Ч���������̼�����������Ҫ�ɷ�H2O2��һ����ɫճ��Һ�壬��ش��������⣺
��1�����з�����H2O2�����ֵ��������������Ϊ��������ȫһ�µ��� ��
A��BaO2+2HCl H2O2+BaCl2
H2O2+BaCl2
B��Ag2O+H2O2 =2Ag+O2+H2O
C��2H2O2 2H2O+O2��
2H2O+O2��
D��H2O2+NaCrO2+NaOH=Na2CrO4 +H2O
��2��������䳣��Һ̬��(N2H4)Ϊȼ�ϣ�Һ̬H2O2Ϊ��ȼ������֪��
N2H4��1��+O2(g)=N2(g)+2H2O(g) ��H=" -" 534 kJ��mol��1
H2O2��1��=H2O��1��+1/2O2(g) ��H=" -" 98.64 kJ��mol��1
H2O��1��=H2O(g) ��H=+44kJ��mol��l
��ӦN2H4��1��+2H2O2��1��=N2(g)+4H2O(g)�ġ�H= ��
�÷�Ӧ�ġ�S= 0(�������<��)��
��3��H2O2��һ�ֲ��ȶ��ֽ�����ʡ�
����ͼ��H2O2��û�д���ʱ��Ӧ�����������仯ͼ������ͼ�ϻ���ʹ�ô����ӿ�ֽ�����ʱ���������ͼ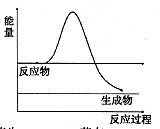
��ʵ��֤ʵ����Na2CO3��Һ�м���H2O2Ҳ�������ݲ�������֪����ʱH2CO3�ĵ��볣���ֱ�ΪKal=4.3��l0��7��Ka2 =" 5.0" ��l0��11 ��Na2CO3��Һ��CO32����һ��ˮ�ⳣ������ʽKhl= ������ʱKhl��ֵΪ ������Na2CO3��Һ��ͬʱ��������Na2CO3�������ʵ�������Һ�¶ȣ���Khl��ֵ
(����С�������ȷ��)��
��4��ij���ױ����˲�ͬ�������Ӽ���Ũ�ȶ�˫��ˮ�������⺣��������Һ��Ӧ���ʵ�Ӱ�죬ʵ������ͼ1��ͼ2��ʾ��
ע������ʵ������¶�Ϊ20�桢w(H2O2)=0��25%��pH=7��12������������ҺŨ��Ϊ8mg��L-l�������½��С�ͼ1������a��H2O2��b��H2O2+Cu2+��c��H2O2+Fe2+��d��H2O2+Zn2+��e��H2O2+Mn2+��ͼ2������f����Ӧʱ��Ϊ1h��g����Ӧʱ��Ϊ2h����ͼ�е��������������������Һ��ճ��(��������Ũ������Һճ�������)��
��������Ϣ��֪����������������� (�����)��
A����������ʹ�ý��ⷴӦ���ʼ���
B���������ӶԸý��ⷴӦ�Ĵ�Ч�ʱ�ͭ���ӵ�
C������������Һճ�ȵı仯�����ɷ�ӳ���併�ⷴӦ���ʵĿ���
D��һ�������£�ͭ����Ũ��һ��ʱ����Ӧʱ��Խ��������������ҺŨ��ԽС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ������Ƿۡ�����غ�ľ̿��һ��������϶��ɵģ���ըʱ�����N2��CO2�ȡ�
��1����ըʱ�Ļ�ѧ����ʽΪ�� ����2�֣�
��2���ںڻ�ҩ��ը�ķ�Ӧ����������������һЩ��Ӧ����Ҳ��������ԭ����д��һ�����ڷ�Ӧ������ԭ���Ļ�ѧ����ʽ�� ����2�֣�
��3�������ڷŵ���������������Ӧ�Ļ�ѧ����ʽ�� ����2�֣�
��4��һ�������������������ǵ������һ�������ڳ����º�����������е��������ϣ���ѧ����ʽΪ�� ����2�֣�������������ˮ��Ӧ����ѧ����ʽΪ�� ����2�֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ǿ���������Ǹ��ֽⷴӦ��һ����Ҫ���ɡ�����ġ�ǿ�ᡱ�������ᡱָ���ǿ�����ܳ������Ե�һЩ���������ʣ�����ࡢ�������������ʽ�εȲ���ķ�ӦҲ�ɸ���������ǿ�����������������ж�����
(1)HA��H2B���������ᣬ�����¹�ϵ��H2B(����)��2A��=B2����2HA����A����HB����B2�����������У����������(H��)����________��
(2)����ǿ���������ʵı����й��⣬�����ܼ��йأ���CH3COOH��HF��Һ������NH3Ӱ��ɷ�����ȫ���롣��Һ����CH3COONa��HCl�D��NaCl��CH3COOH��һ��Ӧ�ܷ���________(��ܡ���)��������____________________��
(3)ijͬѧʵ�鷢�֣���H2S����ͨ��CuSO4��Һ�У����ɺ�ɫ������Ū�������CuS��д���˻�ѧ����ʽ��H2S��CuSO4=CuS����H2SO4��������������������ⲻ�������Ƶ�ǿ��������ǿ��������Ĺ���ì���ˡ������������__________________________________________��
(4)������ԭ��Ӧ��Ҳ�����ƹ��ɣ���ǿ�����������������������ʡ�����ǿ��ԭ������������ԭ�����ʡ����ݴ��ж����з�Ӧ�ܹ���������________(����ĸ���)��
A��FeCl2��Cl2 FeCl3
FeCl3
B��Fe��I2 FeI3
FeI3
C��Fe��CuSO4 FeSO4��Cu
FeSO4��Cu
D��FeCl3��Cu CuCl2��FeCl2
CuCl2��FeCl2
E��FeBr3��Cl2 FeCl2��Br2
FeCl2��Br2
F��FeI2��Br2 FeBr3��I2
FeBr3��I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ԭ��Ӧ��ʵ���ϰ��������ͻ�ԭ�������̡�������һ����ԭ���̵ķ�Ӧʽ��NO3-��4H����3e��=NO����2H2O��KMnO4��Na2CO3��Cu2O��Fe2(SO4)3���������е�һ������(��)��ʹ������ԭ���̷�����
(1)д����������ԭ��Ӧ�Ļ�ѧ����ʽ��________��
(2)��Ӧ������������________��________��
(3)��Ӧ����������״����11.2L���壬��ת�Ƶ��ӵ����ʵ�����________ mol��
(4)�ֳ�ȡ�������ʵļ���Ʒ(���������Ӧ)5.4 g��ǡ����100 mL 1.4 mol��L��1��������Һ��ȫ��Ӧ�������Ʒ�Ĵ���Ϊ________��
(5)��1 mol����ijŨ�����ᷴӦʱ������ԭ��������ʵ������ӣ�ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ĺ���������ҽҩ�������ȷ���������Ҫ����;��
��1���ؾ�ʯ��BaSO4������θ������Ӱ����
��֪�������£�Ksp��BaSO4����1.1��10��10����BaSO4����Һ�м������ᣬ����Һ��pH��2ʱ����Һ��c��Ba2������ __��
��2����������茶���[��NH4��2Fe��SO4��2��6H2O]������������
�ټ��龧���к���NH4+�ķ���Ϊ ��
�ڵ����ʵ���Ũ�ȵ�����ϡ��Һ��
a����NH4��2Fe��SO4��2 b��NH4HSO4
c����NH4�� 2SO4 d����NH4��2SO3��
����c��NH4+���ɴ�С��˳��Ϊ __����ѡ����ĸ����
��3����������أ�K2S2O8������ǿ��������Na2S2O3������ԭ����
��K2S2O8��Һ������MnSO4��Һ��ϣ��ڴ��������£����Թ۲쵽��Һ��Ϊ��ɫ���÷�Ӧ�����ӷ���ʽΪ __��
���ò����缫�����H2SO4��K2SO4�Ļ����Һ�����Ʊ�K2S2O8���������ĵ缫��ӦʽΪ __��������������������Һ��pH�� __���������С�����䡱����
�۲�Ʒ��K2S2O8�ĺ������õ������ⶨ����������Ϊ��ȡ0.3000 g��Ʒ�ڵ���ƿ�У���50 mLˮ�ܽ⣻����4.000 g KI���壨�Թ���������ʹ���ַ�Ӧ����������������Һ�ữ���� __Ϊָʾ������0.1000 mol��L��1 Na2S2O3��Һ�ζ����յ㣨��֪��I2��2S2O32-=2I����S4O62-�����ظ�2�Σ����ƽ�����ı�Һ21.00 mL���ò�Ʒ��K2S2O8����������Ϊ�����ʲ��μӷ�Ӧ�� __����ʽ�����㣩��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ǵؿ��к�����Ϊ�ḻ�ķǽ���Ԫ�أ���Ҫ��������ˮ����������Ca3(PO4)2����ʽ���ڡ����ĵ��ʺͻ������ڹ�ũҵ������������Ҫ��Ӧ�á�
(1)����(P4)����Ca3(PO4)2����̿��SiO2��һ�������·�Ӧ��á�����Ȼ�ѧ����ʽ���£�
2Ca3(PO4)2(s)��10C(s)=6CaO(s)��P4(s)��10CO(g)�� ��H1����3359.26 kJ��mol��1
CaO(s)��SiO2(s)=CaSiO3(s) ��H1����89.61 kJ��mol��1
2Ca3(PO4)2(s)��6SiO2(s)��10C(s)=6CaSiO3(s)��P4(s)��10CO(g)�� ��H3
��H3��________kJ��mol��1��
(2)�����ж������CuSO4��Һ�ⶾ���ⶾԭ���������л�ѧ����ʽ��ʾ��
11P4��60CuSO4��96H2O=20Cu3P��24H3PO4��60H2SO4
60 mol CuSO4�������������ʵ�����________��
(3)����Ҫ������NaH2PO4��Na2HPO4��Na3PO4��ͨ��H3PO4��NaOH��Һ��Ӧ��ã��������ֵķֲ�����(ƽ��ʱij���ֵ�Ũ��ռ������Ũ��֮�͵ķ���)��pH�Ĺ�ϵ����ͼ��ʾ��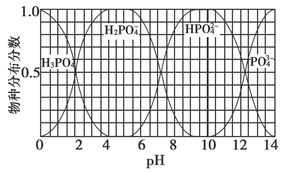
��Ϊ��þ����ܴ���NaH2PO4��pHӦ������________��pH��8ʱ����Һ����Ҫ��������Ũ�ȴ�С��ϵΪ________��
��Na2HPO4��Һ�Լ��ԣ�������Һ�м���������CaCl2��Һ����Һ�������ԣ���ԭ����________(�����ӷ���ʽ��ʾ)��
��4���Ļ������������ף� ���뼾���Ĵ���
���뼾���Ĵ���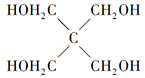 �������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��
�������ʵ���֮��2:1��Ӧʱ���ɻ��һ��������ȼ���м���X�����ͷų�һ���������塣�����Ĵ���X�ĺ˴Ź�����������ͼ��ʾ��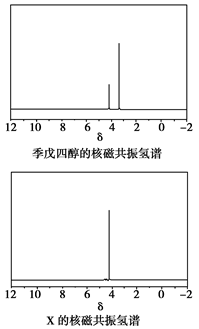
������������______________________(�ѧʽ)��
��X�Ľṹ��ʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о�̼���仯������ۺ����öԴٽ���̼���Ĺ���������Ҫ�����塣���������֪ʶ�о�̼���仯��������ʡ�
��1�����������ҹ��õ绡���ϳɵ�̼�����г����д���̼�����������ʣ�������̼���������������������ᴿ���䷴Ӧ�Ļ�ѧ����ʽΪ��
C+ K2Cr2O7+ �� CO2��+ K2SO4 + Cr2(SO4)3+ H2O
����ɲ���ƽ������ѧ����ʽ��
������������ʽ���õ����ű���÷�Ӧ����ת�Ƶķ�������Ŀ��
��2������ʱ����CO��ԭMgSO4���Ʊ��ߴ�MgO��
��750��ʱ����������к������ʵ���SO2��SO3����ʱ��Ӧ�Ļ�ѧ����ʽ�� ��
����MgO���Ƴɡ�þ���������Ρ���أ���װ��ʾ��ͼ��ͼ1���õ�ط�Ӧ�����ӷ���ʽΪ �� 
 ��
�� 
ͼ1 ͼ2 ͼ3
��3��������̼�ϳɼ״���̼���ŵ��·���CO2ת��Ϊ�״����Ȼ�ѧ����ʽΪ��CO2(g) +3H2(g) CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
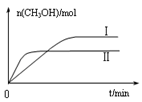
��ȡ��ݵ����CO2��H2�Ļ������(���ʵ���֮�Ⱦ�Ϊ1��3)���ֱ�����¶Ȳ�ͬ���ݻ���ͬ�ĺ����ܱ������У�����������Ӧ����Ӧ��ͬʱ���ü״������������(CH3OH)�뷴Ӧ�¶�T�Ĺ�ϵ������ͼ2��ʾ��������CO2ת��Ϊ�״���Ӧ�ġ�H 0(�>����<������)��
�������ֲ�ͬ�����·�����Ӧ�����CH3OH�����ʵ�����ʱ��仯��ͼ3��ʾ������I��II��Ӧ��ƽ�ⳣ����С��ϵΪK�� KII(����� ����������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com