
�⻯�ƣ�CaH2�������ǵ�ɽ�˶�Ա���õ���Դ�ṩ�����⻯��Ҫ�ܷⱣ�棬һ���Ӵ���ˮ�ͷ�����Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ��ͼ1��ģ����ȡװ�ã�
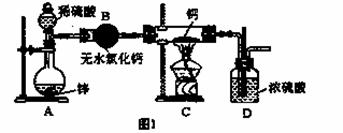
��1��װ��B�������� ��װ��D�������� ��
��2������ͼ1ʵ��װ�ý���ʵ�飬ʵ�鲽�����£����װ�������Ժ�װ��ҩƷ����Һ©�������� ���밴��ȷ��˳���������в������ţ���
�ټ��ȷ�Ӧһ��ʱ�� ���ռ����岢�����䴿��
�۹رշ�Һ©������ ��ֹͣ���ȣ������ȴ
��3��Ϊ��ȷ�Ͻ���װ��C�������Ѿ����Ӧ��B��C֮���ٽ�һװ�ã���װ���м�����Լ��ǣ� ��
��4����ͬѧ���һ��ʵ�飬�ⶨ����ʵ���еõ����⻯�ƵĴ��ȣ������в�����Ԫ�ء�����������ʵ�鲽�裺
����Ʒ�������ڼ���________��Һ���ѧʽ�������衢���ˣ���________����������ƣ�����_______ ����������ƣ��� �ݳ���̼��ơ�
��5����ͬѧ����ע���������⻯�ƺ�ˮ��Ӧ��������ķ������ⶨ����ʵ���еõ����⻯�ƵĴ��ȡ�����ȡ46 mg ���Ƶõ��⻯����Ʒ����¼��ʼʱע������˨ͣ����10.00mL�̶ȴ�����Ӧ����������ȴ����˨����ͣ����57.04mL�̶ȴ�����������������ڱ�״���²ⶨ������ͨ����������Ʒ���⻯�ƵĴ��ȣ� ��
��6�����������һ���⻯�ƴ��ȵIJⶨ������
��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ������С��������ͼ��ʾװ�÷ֱ�������ʵ�飺
��1�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��ɫ��dz����ȴ��ָ���ɫ����ԭ��Һ������________________��Һ������ʱ��Һ�ɺ�ɫ��dz��ԭ���ǣ�_________ ____ __ ��
��2�����Թ���ע��ij��ɫ��Һ�������Թܣ���Һ��Ϊ��ɫ����ȴ��ָ���ɫ�������Һ������________________��Һ������ʱ��Һ����ɫ��Ϊ��ɫ��ԭ���� _________ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2A + B��3C + 4D�ķ�Ӧ�У����б�ʾ�÷�Ӧ�Ļ�ѧ��Ӧ�ٶ�������
A��v (A)��0.5 mol/(L��s) B��v (B)�� 0.3 mol/(L��s)
C��v (C)��0.8 mol/(L��s) D��v (D)��1.0 mol/(L��s)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȡ������²��裺
(1) ���ƴ���Һ�����ѳƺõ�5.0g������������(���ʲ������ᷴӦ)�Ĺ����ռ���Ʒ����1000mL��Һ�����ձ��Ͳ����⣬����Ҫ�õ�����Ҫ������__________��__________��
(2) �ζ����̣�
��ʢװ0.10 mol/L�������ҺӦ��ʹ��_______�ζ��ܣ�
�ڵζ�ʱ˫��Ӧע��۲�___________________________________��
(3) ������ۣ�(ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족 )
�� ������ˮ��ϴ��ƿ���ⶨ���__________��
�� �ڵζ������в�����������Һ������ƿ�⣬�ⶨ���__________��
�� ����ʱ���ζ�ǰ���ӣ��ζ����ӣ��ⶨ���____________��
�� װ��Һ֮ǰ��û���ñ�Һ��ϴ�ζ��ܣ��ⶨ���____________��
(4) �й����ݼ�¼���£�
| �ⶨ��� | ������Һ�����(mL) | ���������Һ�����(mL) | |
| �ζ�ǰ���� | �ζ������ | ||
| 1 | 20.00 | 0.50 | 20.78 |
| 2 | 20.00 | 1.20 | 21.32 |
���㴿�ȣ��ռ���Ʒ�Ĵ�����____________��ȡ����ʵ�����������ƽ��ֵ���м��㣬��д������̣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 D����A��B��C��D��Ϊ10����������C�ǿ�ʹʪ��ĺ�ɫʯ����ֽ���������壬��D������һ����Һ̬
D����A��B��C��D��Ϊ10����������C�ǿ�ʹʪ��ĺ�ɫʯ����ֽ���������壬��D������һ����Һ̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����йؾ����˵���У���ȷ����
A�����Ǿ��й������εĹ��嶼�Ǿ���
B��������ڲ������й̶��۵�
C����̬����O2����̬��S8�����Ƿ��Ӿ���
D���Ȼ��ƺ��Ȼ�虜������Ӿ��壬����ṹ��ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л����˵����ȷ����
A�������һ�ȴ���ֻ��һ��
B���ȷ¼��ܷ���ˮ�ⷴӦ�����ܷ�����ȥ��Ӧ
C����ϩ��CH3—CH = CH2�����Ӿ���˳���칹
D������ϩ���廯�ⷴӦ�Ƶõ�����������������������Ӧ���õIJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����е�̼ԭ����sp3�ӻ���������*��ʾ̼ԭ�ӵ��ӻ��ͼ����е�̼ԭ���ӻ�״̬һ�µ��� �� ��
A��CH3C*H2CH3 B��C*H2��CHCH3
C��CH2��C*HCH3 D��CH2��CHC*H3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ��Ӧ�����ֻ�����Ӧ���͵Ĺ�ϵ��ͼ��ʾ�����л�ѧ��Ӧ������Ӱ���ֵ���

A��4 NH3 +5O2 ��4NO+6 H2O
NH3 +5O2 ��4NO+6 H2O
B��4Fe(OH)2+O2+2H2O=4Fe(OH)3
C��2NaHCO3=Na2CO3+H2O+CO2��
D��Cl2+2NaBr=2NaCl +Br2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com