


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
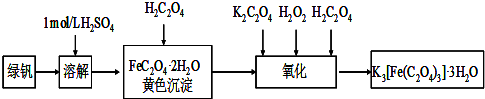
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
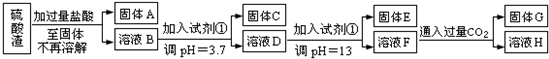
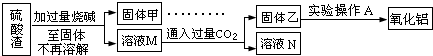
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
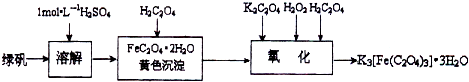
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
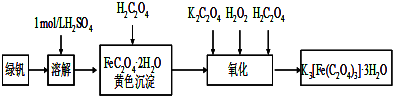
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1������ʵ������У��������ǡ�����������
A��ʵ��������ϩʱ���ھƾ���Ũ����Ļ��Һ�У����뼸Ƭ���Ƭ�����Ȼ�
���ʹҺ���¶�Ѹ������170��
B��ʵ������ȡ����ʱ����ֲ���͡��Ҵ�������������Һ�Ļ������ȳ�ַ�
Ӧ����ȴ����ɴ���˳���̬����
C����֤������ˮ�����ʱ���������������������Һ��ϣ��������Һ��
���á���Һ��ֲ�μ���������Һ
D����֤����ˮ�����ʱ����������Һ�м��뼸��ϡ���ᣬˮԡ5 min������
����������Һ
����2���������

��д�����к��������ŵ����ƣ���_____________________��
�����������л�Ϊͬ���칹���Ϊ__________________��
����ֱ�д������ס��ҡ���������ķ���(ָ����ѡ�Լ�����Ҫ����)��
����ķ����� ��
�����ҵķ����� ��
������ķ����� ��
���밴�ǻ�����ԭ�ӵĻ�������ǿ�������мס��ҡ�����˳�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com