
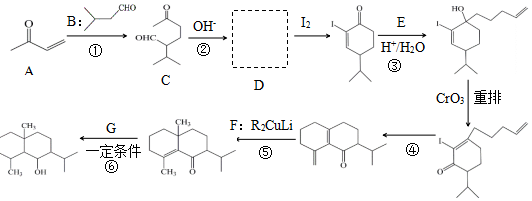
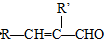 +H2O
+H2O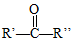 ��
��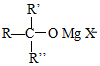 $\stackrel{H+/H_{2}O}{��}$
$\stackrel{H+/H_{2}O}{��}$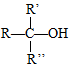

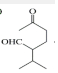 $\stackrel{O{H}^{-}}{��}$
$\stackrel{O{H}^{-}}{��}$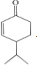 +H2O��
+H2O��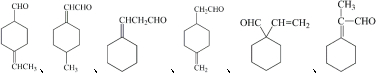
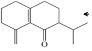
���� C�Ľṹ��ʽΪ ��������֪����ȩʧȥOH-�ã�D�Ľṹ��ʽΪ
��������֪����ȩʧȥOH-�ã�D�Ľṹ��ʽΪ ��������֪����E��
��������֪����E��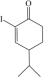 ��H+/H2O������������
��H+/H2O������������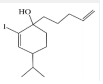 ����E�Ľṹ��ʽΪCH2=CHCH2CH2CH2MgX���ݴ˽��з�����
����E�Ľṹ��ʽΪCH2=CHCH2CH2CH2MgX���ݴ˽��з�����
��8��CH3CH2OH��O2��Cu���������ȵ�����������CH3CHO��CH3CHO��CH3COCH3��ȥOH-����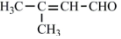 ��
��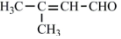 ��H2�����ӳɷ�Ӧ����
��H2�����ӳɷ�Ӧ����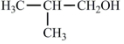 ��
��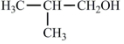 ��O2��Cu���������ȵ�����������Ŀ�����
��O2��Cu���������ȵ�����������Ŀ�����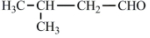 ���ݴ˽��з�����
���ݴ˽��з�����
��� �⣺C�Ľṹ��ʽΪ ��������֪����ȩʧȥOH-�ã�D�Ľṹ��ʽΪ
��������֪����ȩʧȥOH-�ã�D�Ľṹ��ʽΪ ��������֪����E��
��������֪����E�� ��H+/H2O������������
��H+/H2O������������ ����E�Ľṹ��ʽΪCH2=CHCH2CH2CH2MgX��
����E�Ľṹ��ʽΪCH2=CHCH2CH2CH2MgX��
��1������A�Ľṹ��ʽ����A�еĹ������������ʻ���̼̼˫����
�ʴ�Ϊ��̼̼˫����
��2��C�Ľṹ��ʽΪ ��D�Ľṹ��ʽΪ
��D�Ľṹ��ʽΪ ����Ӧ�ڵĻ�ѧ����ʽ��
����Ӧ�ڵĻ�ѧ����ʽ�� $\stackrel{O{H}^{-}}{��}$
$\stackrel{O{H}^{-}}{��}$ +H2O��
+H2O��
�ʴ�Ϊ�� $\stackrel{O{H}^{-}}{��}$
$\stackrel{O{H}^{-}}{��}$ +H2O��
+H2O��
��3�������Ϸ����ã���Ӧ�����Լ�E�Ľṹ��ʽ��CH2=CHCH2CH2CH2MgX��
�ʴ�Ϊ��CH2=CHCH2CH2CH2MgX��
��4����Ӧ�ܷ�����������ȥ��Ӧ���ʻ�ѧ����ʽΪ ��
�� +HI���ʷ�Ӧ�ܵ���һ�ֲ�����HI��
+HI���ʷ�Ӧ�ܵ���һ�ֲ�����HI��
�ʴ�Ϊ��HI��
��5��������֪�����ã���R2CuLi�������·����ӳɷ�Ӧ��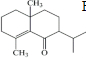 ��
�� ����һ��-CH3����F�Ľṹ��ʽ�ǣ�CH3��2CuLi��
����һ��-CH3����F�Ľṹ��ʽ�ǣ�CH3��2CuLi��
�ʴ�Ϊ����CH3��2CuLi��
��6��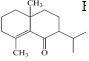 ��
�� ����2��Hԭ�ӣ���
����2��Hԭ�ӣ��� ��
�� �����ӳɷ�Ӧ����FΪH2�������ʽΪH��H��
�����ӳɷ�Ӧ����FΪH2�������ʽΪH��H��
�ʴ�Ϊ��H��H��
��7��D�Ľṹ��ʽ�ܷ���������Ӧ����ȩ����������ṹΪ��Ԫ��״��������3����ԭ�ӣ����������������Ľṹ��ʽΪ ��
��
�ʴ�Ϊ��6�� ��
��
��8��CH3CH2OH��O2��Cu���������ȵ�����������CH3CHO��CH3CHO��CH3COCH3��ȥOH-����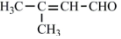 ��
��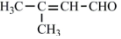 ��H2�����ӳɷ�Ӧ����
��H2�����ӳɷ�Ӧ����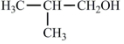 ��
��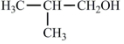 ��O2��Cu���������ȵ�����������Ŀ�����
��O2��Cu���������ȵ�����������Ŀ�����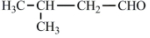 ������CH3CH2OH��CH3COCH3Ϊԭ�ϣ��ϳ�B��·��ΪCH3CH2OH$\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$��CH3CHO$\underset{\stackrel{C{H}_{3}COC{H}_{3}}{��}}{O{H}^{-}}$
������CH3CH2OH��CH3COCH3Ϊԭ�ϣ��ϳ�B��·��ΪCH3CH2OH$\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$��CH3CHO$\underset{\stackrel{C{H}_{3}COC{H}_{3}}{��}}{O{H}^{-}}$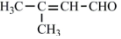 $\stackrel{{H}_{2}}{��}$
$\stackrel{{H}_{2}}{��}$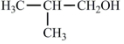 $\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$
$\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$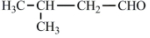 ��
��
�ʴ�Ϊ��CH3CH2OH$\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$��CH3CHO$\underset{\stackrel{C{H}_{3}COC{H}_{3}}{��}}{O{H}^{-}}$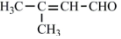 $\stackrel{{H}_{2}}{��}$
$\stackrel{{H}_{2}}{��}$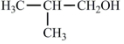 $\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$
$\underset{\stackrel{{O}_{2}/Cu}{��}}{��}$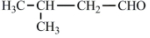 ��
��
���� ���⿼���л�����ƶ���ϳɣ���������л���ķ���ʽ��ṹ���з��������Ҫѧ���������չ����ŵ�������ת�������ؿ���ѧ������������������ѧ������֪ʶǨ��Ӧ�ã��Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
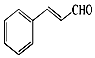 ��Ȼ���е�����ֲ���к���ȩ��������Щ�����������ζ������Ϊֲ������ʹ�ã���Ƥ�к������ȩ���ṹ��ͼ�������к��б���ȩ������˵��������ǣ�������
��Ȼ���е�����ֲ���к���ȩ��������Щ�����������ζ������Ϊֲ������ʹ�ã���Ƥ�к������ȩ���ṹ��ͼ�������к��б���ȩ������˵��������ǣ�������| A�� | ���ȩ�����к������ֹ����ţ�����ʽΪC9H8O | |
| B�� | ���ȩ�����������18��ԭ�ӹ�ƽ�� | |
| C�� | 1 mol���ȩ�������ӳ�ʱ�������4 mol H2 | |
| D�� | ���ȩ�뱽��ȩ����ͬϵ������ܷ���������Ӧ�ͻ�ԭ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
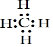 �� B��D��ɵ���ԭ�ӷ��ӵĽṹʽΪO=C=O��
�� B��D��ɵ���ԭ�ӷ��ӵĽṹʽΪO=C=O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1L0.1mol•L-1NH4Cl��Һ�У�NH4+������Ϊ0.1NA | |
| B�� | 2.4gMg��H2SO4��ȫ��Ӧ��ת�Ƶĵ�����Ϊ0.1NA | |
| C�� | ��״���£�2.24LN2��O2�Ļ�������з�����Ϊ0.2NA | |
| D�� | 0.1mol H2��0.1mol I2���ܱ������г�ַ�Ӧ�����������Ϊ0.2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ŀ�� | ���� | |
| A�� | ȡ20.00 mL���� | ��50 mL��ʽ�ζ�����װ�����ᣬ������ʼ����Ϊ30.00mL��ʣ�����������ƿ |
| B�� | ��ϴ������ʵ�������Թ� | ���þƾ���ϴ������ˮ��ϴ |
| C�� | �ⶨ��������ҺpH | �ò�����պȡ��Һ������ʪ���pH��ֽ�� |
| D�� | ����Ũ��Ϊ0.010 mol•L��KMnO4��Һ | ��ȡKMnO4����0.158 g������100 mL����ƿ�У���ˮ�ܽⲢϡ�����̶� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ��ֽ | C�� | ������ | D�� | ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
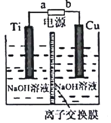
 ȫ��̬����������ܶȸߡ��ɱ��ͣ��乤��ԭ����ͼ��ʾ�����е缫a���ò���ʯīϩ��S8���ϣ���ط�ӦΪ��16Li+xS8=8Li2Sx��2��x��8��������˵��������ǣ�������
ȫ��̬����������ܶȸߡ��ɱ��ͣ��乤��ԭ����ͼ��ʾ�����е缫a���ò���ʯīϩ��S8���ϣ���ط�ӦΪ��16Li+xS8=8Li2Sx��2��x��8��������˵��������ǣ�������| A�� | ��ع���ʱ�������ɷ�����Ӧ��2Li2S6+2Li++2e-=3Li2S4 | |
| B�� | ��ع���ʱ�����·������0.02 mol���ӣ��������ϼ���0.14 g | |
| C�� | ʯīϩ��������Ҫ����ߵ缫a�ĵ����� | |
| D�� | ��س��ʱ��Խ��������е�Li2S2��Խ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1�� HCOONa��NH4Cl ��Һ�������ӵ����ʵ���Ũ��֮�ͣ�ǰ�ߴ��ں��� | |
| B�� | ����ͬŨ�ȵ�NaOH��Һ�ֱ�ζ������pH��Ϊ3��HCOOH��CH3COOH��Һ���յ㣬����NaOH��Һ�������� | |
| C�� | 0.2 mol•L-1 HCOOH �� 0.1 mol•L-1 NaOH �������Ϻ����Һ�У�c��HCOO-��+c��OH-��=c��HCOOH��+c��H+�� | |
| D�� | 0.2 mol•L-1 CH3COONa �� 0.1 mol•L-1����������Ϻ����Һ�У�pH��7����c��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com