
��11�֣���1����ʵ�����������Ũ���������������Ӧ��ȡ��������Ӧ�Ļ�ѧ����ʽΪ��______________________________________________________��
��2��������������Һ�м���������̷�ĩ���Բ����������˷�Ӧ�ж������̵�������_____________��
��3����SO2��CaO��D2��HCl��Na2CO3���ִ������У����ڹ��ۻ��������_________________________�����Ǽ��Լ�����_______________��
��4����֪��2 SO2 + O2 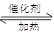 2 SO3 ����һ�ܱ������г���SO2 ��18O2
����Ӧһ��ʱ���18O��������_______________________��
2 SO3 ����һ�ܱ������г���SO2 ��18O2
����Ӧһ��ʱ���18O��������_______________________��
��5�����·�Ӧ��
�� Zn + CuSO4 = ZnSO4 +Cu �� NaOH + HCl = NaCl + H2O
�� 4Al + 3O2 = 2Al2O3 �� Na2CO3 + H2SO4 = Na2SO4 + CO2��+ H2O
���п�����Ƴ�ԭ��ص���_______________��д��ţ���
��1��MnO2
+ 4HCl��Ũ��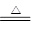 MnCl2 + Cl2��+2H2O��2�֣�
MnCl2 + Cl2��+2H2O��2�֣�
��2����������1�֣� ��3��SO2��HCl��2�֣���D2��1�֣�
��4��O2��SO2��SO3��3�֣� ��5���٢ۣ�2�֣�
����������1�����������ڼ���ʱ��������Ũ������������������ʽΪMnO2 + 4HCl��Ũ��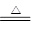 MnCl2 + Cl2��+2H2O��
MnCl2 + Cl2��+2H2O��
��2�����������ֽܷ�����������ˮ���ڴ����������£����Լӿ�ֽ����ʣ���˶�����������������á�
��3��ȫ���ɹ��ۼ��γɵĻ������ǹ��ۻ��������SO2��HCl�ǹ��ۻ���������ƺ�̼���������ӻ������ͬһ�ַǽ����γɵĹ��ۼ��ǷǼ��Լ�������D2���ɷǼ��Լ��γɵĵ��ʡ�
��4����Ϊ�ǿ��淴Ӧ���������������������ͬʱ�����������ַֽ����������Ͷ�������O2��SO2��SO3�о�����18O��
��5��ԭ������е��Ӷ����ƶ�������ֻ��������ԭ��Ӧ������Ƴ�ԭ��أ����٢ۿ��ԡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꺣��ʡ������ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ������
��11�֣���1����ʵ�����������Ũ���������������Ӧ��ȡ��������Ӧ�Ļ�ѧ����ʽΪ��______________________________________________________��
��2��������������Һ�м���������̷�ĩ���Բ����������˷�Ӧ�ж������̵�������_____________��
��3����SO2��CaO��D2��HCl��Na2CO3���ִ������У����ڹ��ۻ��������_________________________�����Ǽ��Լ�����_______________��
��4����֪��2 SO2 + O2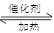 2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________��
2 SO3����һ�ܱ������г���SO2��18O2����Ӧһ��ʱ���18O��������_______________________��
��5�����·�Ӧ��
�� Zn + CuSO4 = ZnSO4 +Cu �� NaOH + HCl =" NaCl" + H2O
�� 4Al + 3O2 = 2Al2O3 �� Na2CO3 + H2SO4 = Na2SO4 + CO2��+ H2O
���п�����Ƴ�ԭ��ص���_______________��д��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ��һ��ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ʵ����
��10�֣���ʵ���������������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʣ��̶�װ����ȥ����ͼ�Т�Ϊ��ȡ������װ�ã����Թ���װ��15mL30%KOH��Һ����������ˮ�У���ȡ����أ����Թ���װ��15 mL 8%NaOH��Һ�������ڱ�ˮ�У�����װ����ɫʯ����Һ��

��1�����Թ�����ȡ�������ƵĻ�ѧ����ʽΪ
��2��ʵ���пɹ۲쵽���Թ�����Һ����ɫ�������±仯����д����
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ ɫ |
������ˮ��Ӧ���ɵ�HClʹʯ����Һ��� |
|
�����Һ��Ϊ��ɫ |
|
|
�����Һ����ɫ��Ϊ ɫ |
|
��3����ʵ����һ�����ԵIJ���֮����Ӧ��θĽ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ ���ͣ������
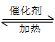 2SO3 ����һ�ܱ������г���SO2 ��18O2 ����Ӧһ��ʱ���18O��������_______________________��
2SO3 ����һ�ܱ������г���SO2 ��18O2 ����Ӧһ��ʱ���18O��������_______________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ������
��ջش�
��1����ʵ���������һ��δ֪��Һ���������___________����ʱ�������֣��ټ���__________������а�ɫ�������֣������ȷ֤ԭ��Һ���д���SO42-����ʵ���п϶������ķ�Ӧ�����ӷ���ʽΪ
______________________��
��2��ʢ���ռ���Һ�IJ����Լ�ƿ�����ò���������Ϊ�˷�ֹ����___________________________��Ӧ���û�ѧ����ʽ��ʾ������ʹƿ����ƿ��ճ��һ��
��3����40mL0.10mol/LBaCl2��Һ�У�����VmL 0.10mol/L H2SO4��Һ����������ʹ������ȫ������Ӧ��Ļ������ˣ�ȡ��Һ��һ�룬����Һ�м���25mL 0.20mol/L NaOH��Һ��ǡ���������ԡ���V=________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com