���㣺ԭ�ӹ���ӻ���ʽ���ӻ������ж�,ԭ�Ӻ�������Ų�,�����ļ���,�������������,��ͬ����Ľṹ��������������������
ר�⣺��ѧ���뾧��ṹ
��������1����ˮʩ��һ�����糡���γ��ȱ���ˮ�����й�������У�˵��ˮ������������ɵ����IJ��غϣ����м��ԣ�ˮ��������ԭ���γ�2��O-H��������2�Թµ��Ӷԣ��ӻ������Ϊ4���ݴ��ж��ӻ���ʽ��
��2���������д��������������з����ԣ�ÿ��ˮ���������ڵ�4��ˮ�����γ������
��3�������þ�̯�����㾧�������ݶ�����̼�Ľṹʽ�жϹ��ۼ������ͺ���Ŀ���ڸɱ���CO
2�������12��
��ͭ���������������ṹ��ѻ���ʽ��ɱ�����������ͬ���������϶Խ������ʵ�3��Cuԭ�����ڣ���Cuԭ�Ӱ뾶Ϊr����������
��4r���������㾧������������ݾ�̯�����㾧����Cuԭ����Ŀ���������㾧����Cuԭ��ʵ��ռ�е�����������ռ�������=������Cuԭ��ʵ��ռ�е�����¾��������
��4�����ݺ��ع������Ų���ͬһ�ܼ��IJ�ͬ���ʱ���ȵ���ռ��һ�������������������ͬ������ԭ����һ��ԭ�ӹ������������2�����ӣ������������෴���NԪ�أ�ԭ�Ӻ��������Ϊ7��д��
��5��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ�����������������ͬһ�����еڢ�A��Ԫ�رȵڢ�A��Ԫ�صĵ�һ�����ܴڢ�A��ȵڢ�A���һ�����ܴݴ˷������
��6��Ԫ��X λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ����ڲ������=2+8+18=28��������������Ϊ2�����Ը�ԭ����30�����ӣ�ΪZnԪ�أ�1mol�� X��NH
3��
4Cl
2�к��еĦļ�����16N
A����
���
�⣺��1����ˮʩ��һ�����糡���γ��ȱ���ˮ�����й�������У�˵��ˮ������������ɵ����IJ��غϣ����м��ԣ�ˮ��������ԭ���γ�2��O-H��������2�Թµ��Ӷԣ��ӻ������Ϊ4����ȡsp
3�ӻ���
�ʴ�Ϊ�����ԣ�sp
3��
��2����������ˮ���ӵĿռ����з�ʽ���������д��������������з����ԣ�ÿ��ˮ���������ڵ�4��ˮ�����γ������
�ʴ�Ϊ��
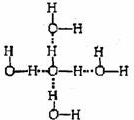
��
��3���ٶ�����̼�ľ����У�������̼���ӷֲ��ھ����Ķ��������λ�ã����к��ж�����̼�ķ�����Ϊ8��
+6��
=4��������̼�ķ��ӽṹΪO=C=C��ÿ�������к���2���Ҽ���2���м����Ըɱ������е�����һ������Ϊ����ԭ�㣬��ͨ���ö�����������Ϊ�����Ὠ����һ����άֱ������ϵ��������ԭ�����Χ������϶����8��������������ÿһ���������϶����Կ���������������ԭ���ϵ�CO
2���ӵȾ����CO
2���ӣ�������ЩCO
2����������ԭ���ϵ�CO
2���ӵľ��벢��������ģ�������ԭ���ϵ�CO
2���������CO
2����Ӧ����ÿһ�������������ϵģ�����12��������CO
2���ӣ�
�ʴ�Ϊ��4��2���Ҽ���2���м���12��
��ͭ�Ķѻ�ģ��Ϊ����ܶѻ�����λ��Ϊ12��ͭԭ��λ�ڶ�������ģ��������̼���Ӿ����ṹ���ƣ�ͭ���������������ṹ���������϶Խ������ʵ�3��Cuԭ�����ڣ���Cuԭ�Ӱ뾶Ϊr���������ⳤ=
��4r=2
r�����������=
(2r)3=16
r
3��������Cuԭ����Ŀ=8��
+6��
=4��������Cuԭ��ʵ��ռ�е����=4��
��r
3�������ռ�������=
=74%��
�ʴ�Ϊ��ͭ��74%��
��4��NԪ�أ�ԭ�Ӻ��������Ϊ7��������ӹ����ʾΪ��

��Nԭ�ӵ������5�����ӣ�Ϊ2s
22p
3��ӦΪ2s��2p���ӣ���2p�����ӦΪ3�������ӣ�Υ�����ع���
�ʴ�Ϊ��

��Υ�����ع���
��5��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ�����������������ArԪ��3s
23p
6��p���Ϊȫ����״̬������ʧȥ���ӣ����Ե�һ���������
�ʴ�Ϊ��Ar��
��6��Ԫ��X λ�ڵ������ڣ����̬ԭ�ӵ��ڲ���ȫ���������ӣ����ڲ������=2+8+18=28��������������Ϊ2�����Ը�ԭ����30�����ӣ�����30�����ӣ�ΪZnԪ�أ�ÿmol�����[X��NH
3��
4]Cl
2�У��Ҽ���Ŀ=��3��4+4��N
A=16N
A��
�ʴ�Ϊ��30��16N
A��
���������⿼�����ʽṹ�����ʣ���Ϊ�ۺϣ��漰�����Ų�ʽ����д�������Ľṹ���ӻ����͵��жϵ�֪ʶ���Ƕ�ѧ��������˼ά�����Ŀ��飬ע�������[Zn��NH3��4]Cl2�У���λ��Ҳ�ǦҼ���Ϊ�״��㣬��Ŀ�Ѷ��еȣ�



 ��
�� ��Nԭ�ӵ������5�����ӣ�Ϊ2s22p3��ӦΪ2s��2p���ӣ���2p�����ӦΪ3�������ӣ�Υ�����ع���
��Nԭ�ӵ������5�����ӣ�Ϊ2s22p3��ӦΪ2s��2p���ӣ���2p�����ӦΪ3�������ӣ�Υ�����ع��� ��Υ�����ع���
��Υ�����ع���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
