
| ||
| �� |
| Br2 |
| ����NaOH��Һ |
| 1.34g |
| 134g/mol |
| 0.448L |
| 22.4L/mol |
| 1.34g |
| 134g/mol |
| 0.448L |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ũ���� |
| �� |
| Br2 |
| ����NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�����ʡ���һ�и߶�4�¶ο���ѧ�Ծ����������� ���ͣ���ѡ��
ij������ӣ���к���һ���ǻ��ᣨ��M��ʾ����M��̼���ṹ��֧��������ʽΪ
C4H6O5��1.34gM��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448L��M��һ�������¿ɷ�������ת����M A
A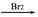 B
B C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
A��M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH
B��B�ķ���ʽΪC4H4O4Br2
C����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��2��
D��C���ʲ���������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ʡ�߶�4�¶ο���ѧ�Ծ��������棩 ���ͣ�ѡ����
ij������ӣ���к���һ���ǻ��ᣨ��M��ʾ����M��̼���ṹ��֧��������ʽΪ
C4H6O5��1.34gM��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448L��M��һ�������¿ɷ�������ת����M A
A B
B C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
C��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ���� �� ��
A��M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH
B��B�ķ���ʽΪC4H4O4Br2
C����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��2��
D��C���ʲ���������ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���ѧ�ڵ�һ���¿���ѧ�Ծ� ���ͣ�ѡ����
ij������ӣ���к���һ���ǻ��ᣨ��M��ʾ����M��̼���ṹ��֧��������ʽΪC4H6O5��
1.34gM��������̼��������Һ��Ӧ�����ɱ�״���µ�����0.448L��M��һ�������¿ɷ�������ת���� ��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ����
�� ��
��M��A��B��C������̼ԭ����Ŀ��ͬ���������й�˵���в���ȷ����
�� ��
A��M�Ľṹ��ʽΪHOOC��CHOH��CH2��COOH
B��B�ķ���ʽΪC4H4O4Br2
C����M�Ĺ��������ࡢ������ȫ��ͬ��ͬ���칹�廹��1��
D��C���ʿ�������ˮ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com