
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?μ�϶�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮
��2013?μ�϶�ģ�������������ʵ���Ҫ���������ʽṹ����ش��������⣮| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C-C | C-N | C-S |
| ����/kJ?mol-1 | 347 | 305 | 259 |
| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ?mol-1 | 786 | 715 | 3401 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ʵ ʵ����� | ʵ������ | �йػ�ѧ����ʽ | ||||||||||||||||
| ��C�м�����Ʒ�걾W�ˣ�D��װ��ҩƷ����Ϊm1�ˣ����Ӻ�������������� | B��E�������ݲ��� B��E�������ݲ��� |
2H2O2
2H2O2
| ||||||||||||||||
| ��A�Ļ����������μ���Һ�� | C�еĺ��ɫ��ĩ��ɺ�ɫ C�еĺ��ɫ��ĩ��ɺ�ɫ |
2Cu+O2
2Cu+O2
| ||||||||||||||||
| ��C���м��ȣ���C��ҩƷ��ַ�Ӧ�ر�A�Ļ�����ֹͣ���ȣ� | �� �� |
�� �� | ||||||||||||||||
| ��ȴ����D������Ϊm2�ˣ� | �� �� |
�� �� |
W-(m2-m1)��
| ||
| W |
W-(m2-m1)��
| ||
| W |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ����12����ϰ���Ի�ѧ�Ծ� ���ͣ������
��12�֣������������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����֪A��BΪ��������Ԫ�أ���ԭ�ӵĵ�һ�����ĵ��������±���ʾ��
| ������/kJ��mol��1 | I1 | I2 | I3 | I4 |
| A | 578 | 1817 | 2745 | 11578 |
| B | 738 | 1451 | 7733 | 10540 |
| ���ۼ� | C��C | C��N | C��S |
| ����/ kJ��mol��1 | 347 | 305 | 259 |

| ���Ӿ��� | NaCl | KCl | CaO |
| ������/kJ��mol��1 | 786 | 715 | 3401 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ�߶�4���¿���ѧ�Ծ� ���ͣ�ʵ����
����ijУ����̽��С������ҵ��ʱ����������ϩ���������ӳɷ�Ӧ���й���Ϣ����õ��йؽṹ�������б����£�����ϩΪ����
|
ϩ �� �� �� �� |
�� �� �� �� |
|
(CH3)2C=CHCH3 |
10.4 |
|
CH3CH=CH2 |
2.03 |
|
CH2=CH2 |
1.00 |
|
CH2=CHBr |
0.04 |
��1���ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������̼̼˫����ȡ���������ࡢ������Ĺ�ϵ��___________________________ ______ ��
��2�����л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________��
A��(CH3)2C= C (CH3)2 B��CH3CH=CHCH3 C��CH2=CH2 D��CH2=CHCl
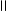 ������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ�
O
������һ����̽��С�������ѧ֪ʶ���������Ҵ���ˮ�����ӡ�̼�ᣨ HO��C��OH��������������ǻ�����ԭ�ӵĻ����ԡ�
O
��1����ͬѧ��һЩ��ѧʵ���ռ��������£�
A�������Ʒֱ���ˮ���Ҵ���Ӧ B�����ӡ�������̼������ֱ�������������Һ��Ӧ
C���������̼ˮ��Һ��������Һ�зֱ�μ���ɫʯ����Һ
D�����������������뵽������Һ�� E������Һ�еμ�FeCl3��Һ
F��������Һ�зֱ�ͨ�������̼�͵μ�����
������˵��ˮ���Ҵ�ǿ���� ������ĸ����ͬ���������̼��ǿ���� ��
��2����ͬѧ������ͬѧ�����������һ����һ������ɵ�ʵ��װ������֤���ᡢ̼��ͱ�����Һ������ǿ������˵�����ǻ�����ԭ�ӵĻ�����˳��������ʵ��װ��ͼ�ش��й����⡣

�� A��Ӧ������������ ��
�� ������������˳���ǣ������֣��������ظ�ʹ�ã� ��
�� ����NaHCO3��Һ������ ��
�� ʵ������й۲쵽�������� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com