
����(Na2S2O3��5H2O)���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ�Na2S2O3��5H2O��Na2S2O3��5H2O�IJ������ʼ��±���
ʵ�����Ʊ������������£�

(1)ʵ�鿪ʼʱ������1 mL��C2H5OH��Ŀ����________
(2)��ҺA�г���Na2S2O3������Na2SO3�⣬����ܴ��ڵ���������������________�������Һ�и����ʵĺ������ܵͣ�����ķ�����________
(3)���˲������õIJ���������________�����˺�������B������������ɫ���ʣ�Ϊ��ȥ����ɫ���ʣ�ϴ������B�IJ���������__________________
(4)�������BʱӦע��ʲô����________��ԭ����__________________
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ԭ�Ӿ������Ǻ�����ѧ��Ӧ��ɫ������Ҫָ�꣬ԭ�Ӿ�������ԭ����������������ԭ�������ʵ���Ŀ��������������������֮�ȡ���������ƾ����׳ƺ���(Na2S2O3?5H2O)����մ����ǻ�ѧ����Ҫ��������ԭ��Ӧ�ĵζ�������ͼ�ǹ�ҵ��ij���Ʊ���������Ƶ�����ʾ��ͼ����֪MΪ��ɫ���崿���ֻ��������Ԫ�أ�������Ԫ��������Ϊ7:4����Ӧ(V)�Ļ�ѧ����ʽΪNa2SO3+S=Na2S2O3��
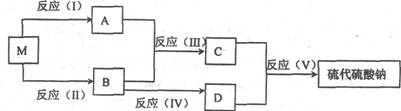
(1)д����Ӧ(I)�����ӷ���ʽ��______________________________________________��
(2)д����Ӧ(��)�Ļ�ѧ����ʽ��_____________________________________________��
(3)��Ӧ(V)�У�����1molNa2S2O3����ʱ��ת�Ƶĵ�������Ϊ_____________________��
(4)��Ӧ(I)�ͷ�Ӧ(II)��Ͷ��M�����ʵ���֮��Ϊ_____________ʱ��������ɫ��ѧ��Ҫ�������ϣ�1molM�������ȡNa2S2O3?5H2O������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
| �������� | ������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
| ��ѧ���� | 43�����ϵĿ������绯�������ֽ� (S2O32+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�
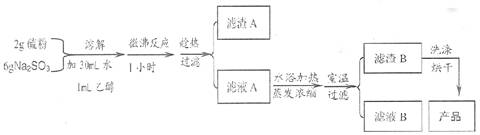
��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������ Na2SO3 �⣬����ܴ��ڵ���������������
�� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11�� )���� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
| �������� | ������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
| ��ѧ���� | 43�����ϵĿ������绯�������ֽ� (S2O32�D+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�

��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������Na2SO3 �⣬����ܴ��ڵ��������������� �� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�廪ʵ��ѧУ2010�������ѧ��3���¿� ���ͣ�ʵ����
(11�� )���� (Na2S2O3•5H2O) ���������ȼ�����Ӱ�����ⶾ������ۺ�����������Һ��п��Ƶ� Na2S2O3•5H2O��Na2S2O3•5H2O�IJ������ʼ��±���
|
�������� |
������ˮ���������Ҵ����۵� 48.2�棻�ڳ�ʪ�Ŀ������׳��� |
|
��ѧ���� |
43�����ϵĿ������绯�������ֽ� (S2O32�D+2H��=S��+SO2��+H2O ) |
ʵ�����Ʊ������������£�

��1��ʵ�鿪ʼʱ������1 mL C2H5OH ��Ŀ����
��2����Һ A �г���Na2S2O3������ Na2SO3 �⣬����ܴ��ڵ��������������� �� �����Һ�и����ʵĺ������ܵͣ�����ķ�����
��3�����˲������õIJ��������� �� ���˺������� B ������������ɫ���ʡ�Ϊ��ȥ����ɫ���ʣ�ϴ������ B �IJ���������
��4��������� B ʱӦע��ʲô���� ��ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com