
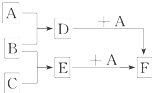
 ��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
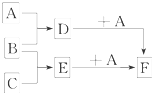 ��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧʱ�ɹ۲쵽��ɫ���棬����K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(15��)��֪A��B��C�����ֳ����Ĺ��廯�����ɫ��Ӧ���Ի�ɫ������A��C�����������ϣ�����������ˮ�У��õ�������Ҳֻ����һ�֣�������ɫ����ζ������D�ų�����A��B��C��D֮�京�����µ�ת����ϵ������������δд����

��1��д�����ʵĻ�ѧʽ��
A �� B �� C ��
��2��д��A��B�����ʵ���֮��Ϊ1��1���ܱ������м��ȷ�����Ӧ�Ļ�ѧ����ʽ��
��
��3��д������E������CO2��Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��6�֣���֪A��B��C�����ֳ����ĵ��ʣ�����AΪ�ճ������г����Ľ�����B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ��K������ˮ����ɫ��ҺE������֮��ת����ϵ��ͼ��ʾ��


��1��д���������ʵĻ�ѧʽ��
B C��������������������
��2��д��D��E�ֱ���A��Ӧ�����ӷ���ʽ��
D+A��
E+A��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ��K������ˮ����ɫ��ҺE.����֮��ת����ϵ��ͼ��ʾ��

(1)д��D��E�ֱ���A��Ӧ�����ӷ���ʽ��
D��A��_______________________________________________________.
E��A��______________________________________________________
(2)д����F�м���NaOH���ڿ����з����������ķ�Ӧ�Ļ�ѧ����ʽ��_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪A��B��C�����ֳ����ĵ��ʣ�����AΪ���壬B��CΪ���壻D�ı�����Һ�����ˮ�м�����У���Һ�ʺ��ɫ��B��C��Ӧ�IJ��K������ˮ����ɫ��ҺE.����֮��ת����ϵ��ͼ��ʾ��
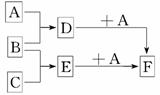
(1)д��D��E�ֱ���A��Ӧ�����ӷ���ʽ��
D��A��_________________________________________________________________.
E��A��___________________________________________________________________.
(2)д����F�м���NaOH���ڿ����з����������ķ�Ӧ�Ļ�ѧ����ʽ��
_______________________________ _______________________________.
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com