
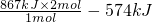 =1160kJ��
=1160kJ��

 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1��좣�PH3����һ����ɫ�о綾�����壬�仹ԭ�����Ȱ���NH3��ǿ����һ��ǿ��ԭ����
�����ˮ�е��ܽ��ԶС�ڰ�������ˮ��ij��Ӧ��ϵ�д����������ʣ�Cu��H2SO4��
CuSO4��PH3��H3PO4��H2O���ش��������⣺
��������Ӧ��ϵ�л�ѧ��Ӧ����ʽΪ ��
��좣�PH3����ˮ�е��ܽ��ԶС�ڰ���ԭ���� ��
 ��2��SO2���ŷ�������������Ҫ���ء�ij��������
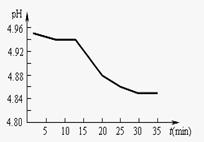
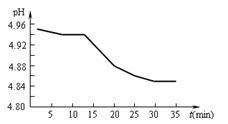
��2��SO2���ŷ�������������Ҫ���ء�ij��������
pH��ʱ��ı仯����ͼ��ʾ�����û�ѧ����ʽ
��ʾ�õ�������pH��ʱ�����Ӷ���С��ԭ��
��
��3��������ˮ�к�����ϸС��������ɼ���ijЩ
����ʹ֮�ۼ��ɽϴ�Ŀ����������������һ
�ֳ�������ʹ������ˮ��������۳������ʣ�
�仯ѧʽΪ ����������ˮ�к�
�����ĵ������ͨ��������Ĥ�ѵ����ս��д���������������ϸ���������½�
NH4������ΪNO3����NH4����2O2��NO3����2H����H2O��Ȼ�����״���NO3���ͼ״�ת��
Ϊ���������塣��д������״���Ӧ�����ӷ���ʽ ��
��4����CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)+4NO2(g) 4NO(g)��CO2(g)+2H2O(g) ��H1����574 kJ��mol��1
CH4(g)+4NO(g) 2N2(g)��CO2(g)+2H2O(g) ��H2
��1molCH4��ԭNO2��N2�����������зų�������Ϊ867kJ����H2�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)좣�PH3����һ����ɫ�о綾�����壬�仹ԭ�����Ȱ���NH3��ǿ����һ��ǿ��ԭ���������ˮ�е��ܽ��ԶС�ڰ�������ˮ��ij��Ӧ��ϵ�д����������ʣ�Cu��H2SO4��CuSO4��PH3��H3PO4��H2O���ش��������⣺ks5u
��������Ӧ��ϵ�л�ѧ��Ӧ����ʽΪ__ ��
 ��좣�PH3����ˮ�е��ܽ��ԶС�ڰ���ԭ����___ ��
��좣�PH3����ˮ�е��ܽ��ԶС�ڰ���ԭ����___ ��
(2)SO2���ŷ�������������Ҫ���ء�ij�������� pH��ʱ��ı仯����ͼ��ʾ�����û�ѧ����ʽ��ʾ�õ�������pH��ʱ�����Ӷ���С��ԭ��
(3)������ˮ�к�����ϸС��������ɼ���ijЩ����ʹ֮�ۼ��ɽϴ�Ŀ����������������һ�ֳ�������ʹ������ˮ��������۳������ʣ��仯ѧʽΪ ��������������ˮ�к������ĵ������ͨ��������Ĥ�ѵ����ս��д���������������ϸ���������½�NH4������ΪNO3����NH4����2O2��NO3����2H����H2O��Ȼ�����״���NO3���ͼ״�ת��Ϊ���������塣��д������״���Ӧ�����ӷ���ʽ ��
(4)��CH4����ԭNOx�������������������Ⱦ�����磺
CH4(g)+4NO2(g) 4NO(g)��CO2(g)+2H2O(g) ��H1����574 kJ��mol��1
CH4(g)+4NO(g) 2N2(g)��CO2(g)+2H2O(g) ��H2
��1mol CH4��ԭNO2��N2�����������зų�������Ϊ867kJ����H2�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com