
 ��ϸͭ����ҪӦ���ڵ�����ϡ������������У���ϸͭ�۵�ij�Ʊ��������£�
��ϸͭ����ҪӦ���ڵ�����ϡ������������У���ϸͭ�۵�ij�Ʊ��������£�
2- 3 |
| ||
| ||
| 1 |
| 2 |
| 1 |
| 8 |
| 1 |
| 2 |
| ||
| ||


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��14�֣���ϸͭ����ҪӦ���ڵ�����ϡ������������С���ϸͭ�۵�ij�Ʊ��������£�
![]()
�Իش��������⣺
��1�����й���[Cu(NH3)4]SO4��˵���У���ȷ����__________��������ĸ��ţ�
A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ��
B��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3����
C��[Cu(NH3)4]SO4�����Ԫ���е�һ��������������Ԫ��
D��[Cu(NH3)4]SO4��������ӵĿռ乹��Ϊ��������
��2��NH4CuSO3�еĽ��������ӵĺ�������Ų�ʽΪ_______________��
��3��SO2�C3 ������S��ԭ�ӵ��ӻ���ʽΪ________�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��___________��
��4��NH3��Һ����ԭ����___________________��
��5����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ���ɴ˿�ȷ����������Ļ�ѧʽ_____��
��6��NH4CuSO3�������ȷ�Ӧ�����ӷ���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��������̨���и߶���ѧ����ĩ��ǰģ�⻯ѧ�Ծ� ���ͣ�ʵ����
��14�֣���ϸͭ����ҪӦ���ڵ�����ϡ������������С���ϸͭ�۵�ij�Ʊ��������£�
�Իش��������⣺
��1�����й���[Cu(NH3)4]SO4��˵���У���ȷ����__________��������ĸ��ţ�
| A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ�� |
| B��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3���� |
| C��[Cu(NH3)4]SO4�����Ԫ���е�һ��������������Ԫ�� |
| D��[Cu(NH3)4]SO4��������ӵĿռ乹��Ϊ�������� |
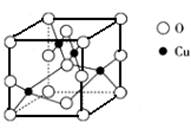 ___________��
___________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�߶���ѧ����ĩģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��14�֣���ϸͭ����ҪӦ���ڵ�����ϡ������������С���ϸͭ�۵�ij�Ʊ��������£�
�Իش��������⣺
��1�����й���[Cu(NH3)4]SO4��˵���У���ȷ����__________��������ĸ��ţ�
A��[Cu(NH3)4]SO4�������Ļ�ѧ�������Ӽ������Լ�����λ��
B��[Cu(NH3)4]SO4����NH3���ӣ���ˮ��Һ��Ҳ����NH3����
C��[Cu(NH3)4]SO4�����Ԫ���е�һ��������������Ԫ��
D��[Cu(NH3)4]SO4��������ӵĿռ乹��Ϊ��������
��2��NH4CuSO3�еĽ��������ӵĺ�������Ų�ʽΪ_______________��
��3��SO2�C3 ������S��ԭ�ӵ��ӻ���ʽΪ________�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��___________��
��4��NH3��Һ����ԭ����___________________��
��5����ͼ��ͭ��ij��������ľ����ṹʾ��ͼ���ɴ˿�ȷ����������Ļ�ѧʽ_____��
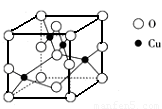
��6��NH4CuSO3�������ȷ�Ӧ�����ӷ���ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ĵ�ʡ�ɶ���������ѧ�߶����ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������

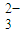 ������S��ԭ�ӵ��ӻ���ʽΪ______�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��______��
������S��ԭ�ӵ��ӻ���ʽΪ______�����以Ϊ�ȵ������һ�ַ��ӵķ���ʽ��______��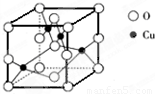
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com