
(8��)������(HNO2)��һ�ֱȴ�����ǿ�����ᣬ�ܲ��ȶ�����������������ԭ��Ӧ���ֽ⡣
(1)�����£��������ʵ�����NO��NO2ͨ��ˮ�У����Ƶ�HNO2����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________.
(2)NO�������������л�ԭ�ԣ������������ԭ��������Һ��pH�Ĺ�ϵ���±���ʾ��
| pH��Χ | ����7 | С��7 |
| ���� | NO | NO��N2O��N2�е�һ�� |
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
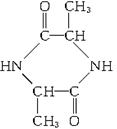
(1)д��A��B�Ľṹ��ʽ��A______________________��B_______________________��
(2)д���йصĻ�ѧ����ʽ��
C![]() E___________________________________________________________��
E___________________________________________________________��
C![]() D___________________________________________________________��
D___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)������(HNO2)��һ�ֱȴ�����ǿ�����ᣬ�ܲ��ȶ�����������������ԭ��Ӧ���ֽ⡣
(1)�����£��������ʵ�����NO��NO2ͨ��ˮ�У����Ƶ�HNO2����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________.
(2)NO�������������л�ԭ�ԣ������������ԭ��������Һ��pH�Ĺ�ϵ���±���ʾ��
| pH��Χ | ����7 | С��7 |
| ���� | NO | NO��N2O��N2�е�һ�� |
���ڼ��������£�NaNO2��Һ��NaClO��Һ��Ӧ�����ӷ���ʽΪ
_________________________________________________________.
����HNO2������ᷴӦʱ�����ʵ���֮��1��2���У���I����������I2��������к���������Ϊ__________(�ѧʽ).
(3)���䶳��NaNO2��Һ�м����ͨ���������ʣ������Ƶ�HNO2����________(�����).
a��ϡH2SO4���� b��ϡHCl c��CO2 d��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ�����и����������¿���ѧ�Ծ��������棩 ���ͣ������
(8��)������(HNO2)��һ�ֱȴ�����ǿ�����ᣬ�ܲ��ȶ�����������������ԭ��Ӧ���ֽ⡣
(1)�����£��������ʵ�����NO��NO2ͨ��ˮ�У����Ƶ�HNO2����Ӧ�Ļ�ѧ����ʽΪ__________________________________________________________.
(2)NO�������������л�ԭ�ԣ������������ԭ��������Һ��pH�Ĺ�ϵ���±���ʾ��
|
pH��Χ |
����7 |
��7 |
|
���� |
NO |
NO��N2O��N2�е�һ�� |
���ڼ��������£�NaNO2��Һ��NaClO��Һ��Ӧ�����ӷ���ʽΪ
_________________________________________________________.
����HNO2������ᷴӦʱ�����ʵ���֮��1��2���У���I����������I2��������к���������Ϊ__________(�ѧʽ).
(3)���䶳��NaNO2��Һ�м����ͨ���������ʣ������Ƶ�HNO2����________(�����).
a��ϡH2SO4���� b��ϡHCl c��CO2 d��SO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ�߶���ѧ�����п��Ի�ѧ���� ���ͣ�ѡ����
���и�ѡ��������������������������ȵ��ǣ�
A���Ǽ�(-CH2OH)�ͼ�����(CH3O-) B��������(HNO2)���������(NO2-)
C������(-NO2)�Ͷ�������(NO2) D���ǻ�(-OH)��������(OH��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com