
 ��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã�
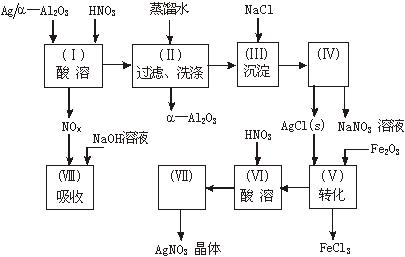
��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã� �������Ҳ��������ᣬ�ô�������ʵ����ͼ��ʾ�����е�ת����ӦΪ��
�������Ҳ��������ᣬ�ô�������ʵ����ͼ��ʾ�����е�ת����ӦΪ��
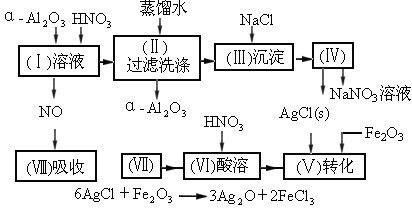
�Ķ�����ʵ�����̣����������գ�
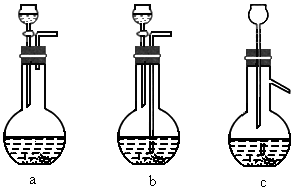
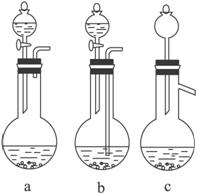
(1)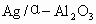 �����ܽ�Ӧ��ѡ��װ��___________(ѡ��a��b��c)��
�����ܽ�Ӧ��ѡ��װ��___________(ѡ��a��b��c)��
(2)��ʵ�����(��)�����������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ___________��
(3)ʵ�����(��)���貣������Ϊ___________(��д����)��
(4)��ʵ�����(��)��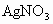 �FҺ���
�FҺ���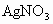 ������Ҫ���е�ʵ���������Ϊ��_________________________��
������Ҫ���е�ʵ���������Ϊ��_________________________��
a������b��������c�����ա�d�����ˡ�e����ȴ�ᾧ
(5)��֪��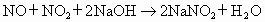

NO��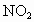 �Ļ���������ɿɱ�ʾΪ
�Ļ���������ɿɱ�ʾΪ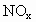 ���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ___________��
���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ___________��
(6)��֪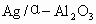 ��Ag��������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ__________��_________��
��Ag��������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ__________��_________��
|
���� (1)a��(2)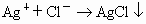 ��(3)©�����ձ���������(4)b��e��d��(5)c��(6)������������ ��(3)©�����ձ���������(4)b��e��d��(5)c��(6)������������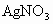 �������� ��������
���� (1)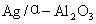 ��ϡ�����ܽ�����У�һ������Ҫ�÷�Һ©������Һ����������һ����Ҫ������NO�����ų������ʵ��Ļ�������������a��b��c����װ�ã�������������a��(2)����ˮ����һ������ ��ϡ�����ܽ�����У�һ������Ҫ�÷�Һ©������Һ����������һ����Ҫ������NO�����ų������ʵ��Ļ�������������a��b��c����װ�ã�������������a��(2)����ˮ����һ������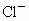 ���� ����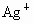 �ᷢ����Ӧ����AgCl������(3)�����̷�����ʵ�����(��)��AgCl���ˣ������Ҫ��������Ҫ��©�����ձ��������ȣ�(4)���� �ᷢ����Ӧ����AgCl������(3)�����̷�����ʵ�����(��)��AgCl���ˣ������Ҫ��������Ҫ��©�����ձ��������ȣ�(4)����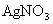 ��Һ��� ��Һ���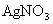 ������Ҫ��������Ũ��(ע����������ڵĻ����£�������ˮ���ܵ�����)��Ȼ����ȴ�ᾧ�����ͨ�����˼������������壮(5)����Ϣ��֪�� ������Ҫ��������Ũ��(ע����������ڵĻ����£�������ˮ���ܵ�����)��Ȼ����ȴ�ᾧ�����ͨ�����˼������������壮(5)����Ϣ��֪��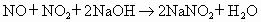 �� ��
 ��1������������ѡ�ֻ��x��1.5ʱ��������������(6)֪�������������� ��1������������ѡ�ֻ��x��1.5ʱ��������������(6)֪��������������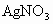 ���������ɼ���Ag�Ļ����ʣ� ���������ɼ���Ag�Ļ����ʣ� |


 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
 ��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã�
��ʯ�ͻ�ѧ��ҵ��һ����Ҫ����������Ag������ã� �������Ҳ��������ᣬ�ô�������ʵ����ͼ��ʾ�����е�ת����ӦΪ��
�������Ҳ��������ᣬ�ô�������ʵ����ͼ��ʾ�����е�ת����ӦΪ��
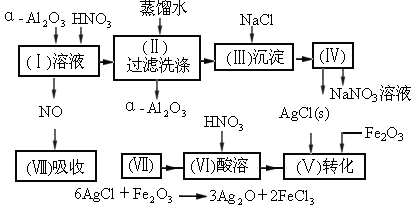
�Ķ�����ʵ�����̣����������գ�
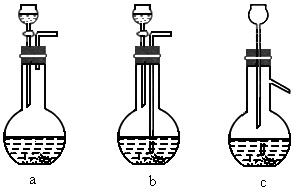
(1)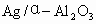 �����ܽ�Ӧ��ѡ��װ��___________(ѡ��a��b��c)��
�����ܽ�Ӧ��ѡ��װ��___________(ѡ��a��b��c)��
(2)��ʵ�����(��)�����������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ___________��
(3)ʵ�����(��)���貣������Ϊ___________(��д����)��
(4)��ʵ�����(��)�� �FҺ���
�FҺ��� ������Ҫ���е�ʵ���������Ϊ��_________________________��
������Ҫ���е�ʵ���������Ϊ��_________________________��
a������b��������c�����ա�d�����ˡ�e����ȴ�ᾧ
(5)��֪��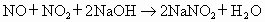

NO��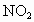 �Ļ���������ɿɱ�ʾΪ
�Ļ���������ɿɱ�ʾΪ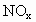 ���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ___________��
���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ___________��
(6)��֪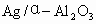 ��Ag��������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ__________��_________��
��Ag��������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ__________��_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
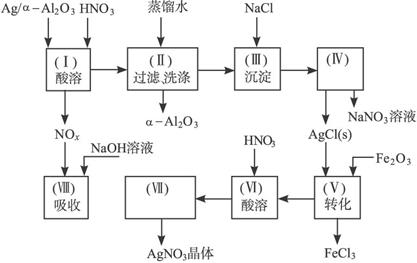
ͼ15-18
�Ķ�����ʵ�����̣����������գ�
��1��Ag/��Al2O3�����ܽ�Ӧ��ѡ��װ��____________________________��ѡ��a��b��c����
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽΪ_________________________________��
��3��ʵ��������������貣������Ϊ_________________����д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ��________��
a.���� b.���� c.���� d.���� e.��ȴ�ᾧ
��5����֪��NO+NO2+2NaOH====2NaNO2+H2O��
2NO2+2NaOH====NaNO3+NaNO2+H2O��
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ________________��
a.x��1.5 b.x=1.2 c.x��1.5
��6����֪Ag/��Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ_____��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���Ϻ��߿����� ���ͣ�ʵ����

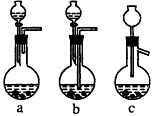
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ķ�����ʵ�����̣����������գ�
��1��Ag/����Al2O3�����ܽ�Ӧ��ѡ��װ��������ѡ��a��b��c����
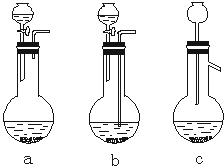
��2����ʵ������������������ˮ��������ˮ����ϴ�ӣ����ᷢ����ѧ��Ӧ�����ӷ���ʽ
����������������������������������
��3��ʵ��������������貣������Ϊ����������������д���֣���
��4��ʵ�������������AgNO3��Һ���AgNO3������Ҫ���е�ʵ���������Ϊ������������������ѡ�۷֣���
��a������b�������� ��c�����ա� ��d�����ˡ� ��e����ȴ�ᾧ
��5����֪��NO+NO2+2NaOH![]() 2NaNO2+H2O��
2NaNO2+H2O��
2NO2+2NaOH![]() NaNO3+NaNO2+H2O
NaNO3+NaNO2+H2O
NO��NO2�Ļ���������ɿɱ�ʾΪNOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵΪ
��������������
��a��x��1.5�� ��b��x=1.2�� ��c��x��1.5
��6����֪Ag/����Al2O3��Ag������������������Ag�Ļ����ʣ�������֪����ʵ������Ϊ��������������
��������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com