




| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 cC��g��+ dD��g����������ͼʾ�ش�
cC��g��+ dD��g����������ͼʾ�ش�

| A���٣��ڣ��� | B���٣��ڣ��� | C����=��=�� E���٣���=�� |
| D����=�ۣ��� F���٣���=�� G����=�ڣ���H����=�ڣ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��N4���۵��P4�� |
| B��1 mol N4����ת��ΪN2ʱҪ����724 kJ���� |
| C��N4��N2��ͬϵ�� |
| D��1 mol N4����ת��ΪN2ʱҪ�ų�724 kJ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��������������������������ʱ���������ȷ�Ӧ |
| B��ȼú������ֱ�Ӱѻ�ѧ��ת��Ϊ���� |
| C����Ҫ���Ȳ��ܽ��еĻ�ѧ��Ӧ��һ�������ȷ�Ӧ |
| D����ѧ��Ӧ�����е������仯�����������غ㶨�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����Ƭ��ϡH2SO4��Ӧ��ȡH2 |
| B��̼��Ƹ��·ֽ�������ƺͶ�����̼ |
| C���������������������ֽ� |
| D���������غ������к� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������� | B�����ʺ����� | C�����ʵ��� | D�����ʵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B���ڢ� | C���٢ܢ� | D���ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����6a+5d��4c��12b��kJ��mol-1 |
| B����4c+12b��6a��5d��kJ��mol-1 |
| C����4c+12b��4a��5d��kJ��mol-1 |
| D����4a+5d��4c��12b��kJ��mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
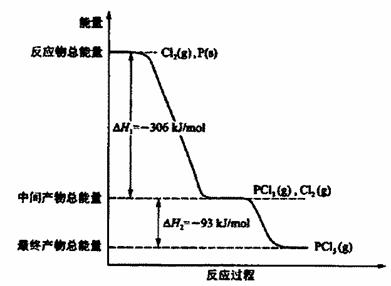
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com