�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���
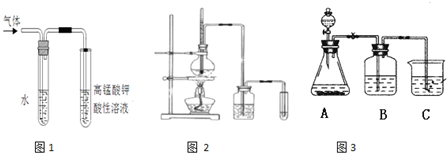
��Ӧ����
ȡ��
ȡ��
��ijͬѧȡ������������NaOHˮ��Һ��Ӧ��Ļ����Һ�������еμ�AgNO
3 ��Һ�����ȣ�����������������ͬѧ�ɴ˵ó���������NaOHˮ��Һ��Ӧ���������廯�ƣ�����Ϊ�Ƿ������ԭ��
������û���������к�����������Һ
������û���������к�����������Һ
��
��2��д����������NaOH�Ҵ���Һ�еķ�Ӧ����ʽ
CH
2BrCH
3+NaOH
CH
2=CH
2��+H
2O+NaBr
CH
2BrCH
3+NaOH
CH
2=CH
2��+H
2O+NaBr
��Ӧ����
��ȥ��Ӧ
��ȥ��Ӧ
����Ӧ�����ɵ����������ͼ1��ʾװ�ü��飬������
��Һ��ɫ
��Һ��ɫ
��ˮ��������
�����Ҵ�
�����Ҵ�
�����������������Һ�⣬��������
������Ȼ�̼��Һ
������Ȼ�̼��Һ
�������ɵ����壬��ʱ���б�Ҫ��������ͨ��ˮ����
û��
û��
����С���û�С���
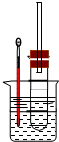
ͼ2��ʵ��������ϩ�����������ʵ�װ��ͼ����ش�
��1��д��Բ����ƿ�з�Ӧ�ķ���ʽ
����Ӧ����
��ȥ��Ӧ
��ȥ��Ӧ
��
��2����ƿ�л��Һ��ڣ�������ijЩ���������壬д��������������Ļ�ѧ����ʽ��
C+2H
2SO
4��Ũ��
CO
2��+2SO
2��+2H
2O
C+2H
2SO
4��Ũ��
CO
2��+2SO
2��+2H
2O
��
��3��Ϊ�˼�����ϩ�����ɣ��Թ���Ӧʢ��
���������Һ����������Ȼ�̼��Һ��
���������Һ����������Ȼ�̼��Һ��
������Ϊ
��Һ��ɫ
��Һ��ɫ
��
��4��ϴƿ��ʢ�ŵ��Լ�Ϊ
����������Һ
����������Һ
������
����SO2CO2
����SO2CO2
ijѧϰС��ͬѧΪ��ȷ�����ᡢ���ӡ�̼�������ǿ�����������ͼ3��ʾ��װ��ͼ����ʵ�飺
��1����ƿ��װij�������Σ�д��A�з�Ӧ�����ӷ���ʽ��
CO32-+2H+=H2O+CO2��
CO32-+2H+=H2O+CO2��
��
��2��C�е�����Ϊ
��ɫ����
��ɫ����
��д��C�з�Ӧ�Ļ�ѧ����ʽ��
C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
C6H5ONa+CO2+H2O��C6H5OH+NaHCO3��
��3��B�е��Լ�Ϊ
����̼��������Һ
����̼��������Һ
������
����CO2�е�HCl
����CO2�е�HCl
��

 �������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ���ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ���ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
 �������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
 �������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����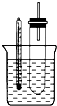 �������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
�������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ�ã�����̨���ƾ����ԣ���֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����