
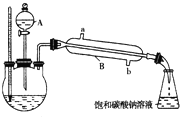
 ��֪�����ʾ���ݣ�
��֪�����ʾ���ݣ�| ���� | �۵㣨�棩 | �е㣨�棩 | �ܶȣ�g?cm-3�� |
| �Ҵ� | -117.3 | 78.5 | 0.79 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | - | 338.0 | 1.84 |
 CH3COOC2H5+H2O��
CH3COOC2H5+H2O�� CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��| 30g |
| 60g/mol |
| 46g |
| 46g/mol |
 CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ���������
CH3COOCH2CH3+H2O���ɴ˿�֪�Ҵ��������� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O

 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ȼ���ȣ�kJ?mol-1�� |
| H2��g�� | 285.8 |
| CO��g�� | 283.0 |
| CH3CH2OH��l�� | 1365.5 |
| �����ǣ�s�� | 2800 |
| ��� | A | B | C | D | E |
| ��ˮ���� | ������ˮ | ӡȾ | ��� | ��ֽ | ���Ṥҵ��ˮ |
| CODֵ��mg/L�� | 520 | 870 | 20 | 960 | 120 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | Al | A12O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565 |
| �е�/�� | 2467 | 2980 | 2750 | ---- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| T/��t/min pH | 3.0 | 4.0 | 5.0 | 6.0 | 20 | 301 | 231 | 169 | 58 | 30 | 158 | 108 | 48 | 15 | 50 | 31 | 26 | 15 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ԭ�� | N | S | O | Si |
| r/10-10m | 0.75 | 1.02 | 0.74 | 1.17 |
| A��1.10��10-10 m |
| B��0.80��10-10 m |
| C��1.20��0-10 m |
| D��0.70��10-10 m |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com