
��13�֣�1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��NH ��Fe2+��Fe3+ ��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��AlO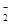 |

| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n(Cl-)[��Դ:ѧ+��+��] | 1.25mol | 1.5mol | 2mol |
| n(Br-) | 1.5mol | 1.4mol | 0.9mol |
| n(I-) | a mol | 0 | 0 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��NH4+��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��AlO2- |
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n��Cl-�� | 1.25mol | 1.5mol | 2mol |
| n��Br-�� | 1.5mol | 1.4mol | 0.9mol |
| n��I-�� | a mol | 0 | 0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 1Lij�����Һ�����ܺ��е��������±��� 1Lij�����Һ�����ܺ��е��������±���
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��13�֣�1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��NH��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��AlO |
��1��������Һ����μ���NaOH��Һ���������������ʵ�����n�������NaOH��Һ�������V���Ĺ�ϵ��ͼ��ʾ�������Һ��һ�������е�������_________��
��2��BC�����ӷ���ʽΪ ��
��3��V1��V2�� V3�� V4֮��Ĺ�ϵ ��
��4������⣬����Һ�л����д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ������
Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��������ش��������⣺
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n(Cl-) | 1.25mol | 1.5mol | 2mol |
| n(Br-) | 1.5mol | 1.4mol | 0.9mol |
| n(I-) | a mol | 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ2.8Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ___________________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��) 1Lij�����Һ�����ܺ��е��������±���
| ���ܴ������е������� | H+��K+��Mg2+��Al3+��NH��Fe2+��Fe3+ |
| ���ܴ������е������� | Cl-��Br-��I-��CO32-��AlO |
�� ������Һ����μ���NaOH��Һ���������������ʵ�����n��
�����NaOH��Һ�������V���Ĺ�ϵ����ͼ��ʾ��
�����Һ��һ�������е�������_________��
(2)BC�����ӷ���ʽΪ ��
(3)V1 ��V2��V3�� V4֮��Ĺ�ϵ ��
(4)����⣬����Һ�л����д�����Cl-��Br-��I-������1L�û����Һ��ͨ��һ������
Cl2����Һ��Cl-��Br-��I-�����ʵ�����ͨ��Cl2���������״�����Ĺ�ϵ���±���ʾ��
������ش��������⣺
| Cl2���������״���� | 2.8L | 5.6L | 11.2L |
| n(Cl-) | 1.25mol | 1.5mol | 2mol |
| n(Br-) | 1.5mol | 1.4mol | 0.9mol |
| n(I-) | a mol | 0 | 0 |
�ٵ�ͨ��Cl2�����Ϊ2.8Lʱ����Һ�з�����Ӧ�����ӷ���ʽΪ___________________��
��ԭ��Һ��Cl-��Br-��I-�����ʵ���Ũ��֮��Ϊ____________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com