

���� ������FeS2�����õ���������ӣ������Ȼ�����Һ�������ᱵ�����������ˡ�ϴ�ӡ����������ƽ�Ƶ����ᱵ������������SԪ���غ�ɼ�����������FeS2������
������FeS2���������õ������ӣ���������������Һ�������������������ˡ�ϴ�Ӻ��������м��ȷֽ�����������������ƽ������������������������Ԫ���غ���������FeS2������
������ԭ������Ϊ�����������Ƴ�100.00mL������Һ���õζ�����ȡ25.00mL������Һ��������0.1mol/LKMnO4����Һ�ζ�������������ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�����������Ԫ�غ���Ԫ���غ���㺬����
��1���ж���Һ��SO42-�����Ƿ������ȫ����ȡ�ϲ���Һ�μ�BaCl2��Һ���۲����������ɣ�
��2���÷����������ʯ�е�Fe���������ֲⶨ�������ƫ�ߣ�˵���������Ĺ��������ƫ���ݹ�������ʽ��з����������Ĺ��������ߣ����½��ƫ�ߣ��ʿ��ܵ�ԭ���У���Fe��OH��3��������������������� �ڹ���ϴ��ʱδ��ֽ�����������ϴȥ ��Fe��OH��3���ղ���֣�δ��ȫת��ΪFe2O3��
��3�����������Һ��������Һ�����������������������ӣ�
��4��1.60g��ʯ�к���n��S��=n��SO42-��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O����Ϸ�Ӧ������ϵ���㣬
����n��Fe2+��=5n��MnO4-����4=5��0.1mol/L��0.0075L��4=0.015mol
���У���Ԫ���غ�n��FeS��+n��FeS2��=0.015mol
��Ԫ���غ㣺n��FeS��+2n��FeS2��=0.02mol
����õ��������ʵ���֮�ȣ�
��� �⣺��1���ж���Һ��SO42-�����Ƿ������ȫ����ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ��
�ʴ�Ϊ��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ��
��2���÷����������ʯ�е�Fe���������ֲⶨ�������ƫ�ߣ�˵���������Ĺ��������ƫ����ϴ�Ӳ���֡����ղ���ֶ��������������ƫ��ԭ�������Fe��OH��3��������������������ʣ�����ϴ��ʱδ��ֽ�����������ϴȥ����Fe��OH��3���ղ���֣�δ��ȫת��ΪFe2O3��
�ʴ�Ϊ����Fe��OH��3��������������������ʣ��ڹ���ϴ��ʱδ��ֽ�����������ϴȥ����Fe��OH��3���ղ���֣�δ��ȫת��ΪFe2O3��
��3���ζ��������Ǹ��������Һ��������Һ�����������������������ӣ���Ӧ�����ӷ���ʽΪ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O��
��4��1.60g��ʯ�к���n��S��=n��SO42-��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��
MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O����Ϸ�Ӧ������ϵ���㣬
����n��Fe2+��=5n��MnO4-����4=5��0.1mol/L��0.0075L��4=0.015mol
���У���Ԫ���غ�n��FeS��+n��FeS2��=0.015mol
��Ԫ���غ㣺n��FeS��+2n��FeS2��=0.02mol
n��FeS��=0.01mol��n��FeS2��=0.005mol��
��ÿ�ʯ��FeS2��FeS��ֵΪ��n��FeS����n��FeS2��=0.01��0.005=2��1��
�ʴ�Ϊ��2��1��
���� ���⿼�鶨��ʵ��Ļ�����������������������й����⣬ע�����֪ʶ�ͻ�ѧ�����������������գ���Ŀ�Ѷ��еȣ�


 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
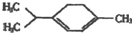
| A�� | 5�� | B�� | 6�� | C�� | 7�� | D�� | 8�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
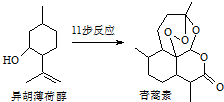 2015��ŵ��������ѧ��ҽѧ����һ�������ҹ�ҩ�ﻯѧ����ߺߺ���Ա�����������ű����ҩ�����غ�˫�������أ���������ɴ�Ϊ��ʼԭ�����˹�ȫ�ϳ������ص�;��֮һ����ͼ��������˵����ȷ���ǣ�������
2015��ŵ��������ѧ��ҽѧ����һ�������ҹ�ҩ�ﻯѧ����ߺߺ���Ա�����������ű����ҩ�����غ�˫�������أ���������ɴ�Ϊ��ʼԭ�����˹�ȫ�ϳ������ص�;��֮һ����ͼ��������˵����ȷ���ǣ�������| A�� | ������ɴ��ķ���ʽΪC10H12O | |
| B�� | ��������ȡʱ����ͨ�����Ȼ�Ӽ��ܽ�ķ���������ȡ�� | |
| C�� | ������������ˮ�����������л��ܼ� | |
| D�� | ������ɴ��ɷ�����ȥ��Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
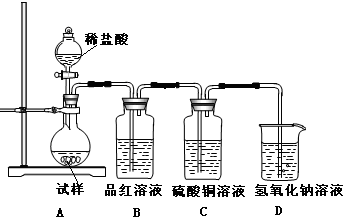
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����о�����ǦԪ�� | B�� | �����в���Ϊ���Ӻ�ǦԪ�ص����� | ||
| C�� | �����к�ǦԪ����һ��ָ�귶Χ�� | D�� | ���϶�����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+��Cl-��Al3+��Ag+ | B�� | $NH_4^+$��Mg2+��$NO_3^-$��$SO_4^{2-}$ | ||
| C�� | K+��Ca2+��$NO_3^-$��$CO_3^{2-}$ | D�� | $HCO_3^-$��Na+��K+��$SO_4^{2-}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
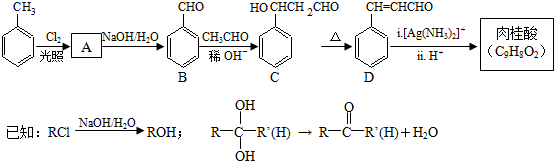
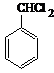 ��
��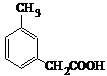 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
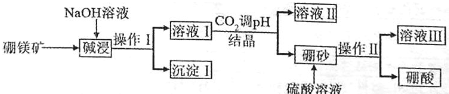
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com