
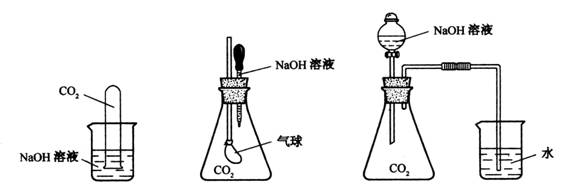
ЃЈ10ЗжЃЉЮЊЬНОПCO2гыЙ§СПЕФNaOHШмвКШЗЪЕЗЂЩњСЫЛЏбЇЗДгІЃЌМзЁЂввЁЂБћШ§ЮЛЭЌбЇЩшМЦСЫЯТСаШ§жжЪЕбщзАжУЃЌЧыЛиД№ЯТСаЮЪЬтЃК
Мз вв Бћ
ЃЈ1ЃЉЧыаДГіCO2гыЙ§СПЕФNaOHЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК ЁЃ
ЃЈ2ЃЉбЁдёШЮвтвЛжжЪЕбщзАжУЃЌМђЪідЄМЦГіЯжЕФЪЕбщЯжЯѓЃЌНтЪЭВњЩњИУЪЕбщЯжЯѓЕФдвђЁЃФубЁдёЕФЪЕбщзАжУЪЧ ЃЌЪЕбщЯжЯѓЪЧ ЁЃ НтЪЭВњЩњИУЪЕбщЯжЯѓЕФдвђ ЁЃ
ЃЈ3ЃЉМзЁЂввЁЂБћЭЌбЇЩшМЦЕФШ§ИіЗНАИжаЃЌгавЛИіЗНАИдкЪЕМЪВйзїжаПЩааадКЭАВШЋадДцдкЮЪЬтЃЌИУЗНАИЪЧ ЁЃЃЈЬюМзЁЂввЛђБћЃЉ
ЃЈ4ЃЉЧыЩшМЦвЛИіЪЕбщМьбщЩњГЩЕФВњЮяжаКЌгаNa2CO3ЁЃЃЈМђЪіЫљгУЪдМСЁЂЪЕбщЯжЯѓКЭНсТлЁЃЃЉ
ЃЈ1ЃЉCO2 + 2NaOH ===Na2CO3 + H2O ЃЈ2ЗжЃЉ
ЃЈ2ЃЉМз ЯжЯѓЃКЪдЙмФквКУцЩЯЩ§ЃЌЩеБФкЕФвКУцЯТНЕ
двђЃКЖўбѕЛЏЬМКЭЧтбѕЛЏФЦШмвКЗДгІЃЌЪЙЪдЙмФкЦјЬхбЙЧПМѕаЁЃЌвКЬхБЛбЙШыЪдЙмжа
бЁ вв ЯжЯѓЃКЦјЧђеЭДѓЁЃ
двђЃКЖўбѕЛЏЬМКЭЧтбѕЛЏФЦШмвКЗДгІЃЌЪЙзЖаЮЦПФкЦјЬхбЙЧПМѕаЁЃЌДѓЦјбЙЪЙЦјЧђеЭДѓ
бЁ Бћ ЯжЯѓЃКЩеБФкЕФЫЎСїШызЖаЮЦПжаЁЃ
двђЃКЖўбѕЛЏЬМКЭЧтбѕЛЏФЦШмвКЗДгІЃЌЪЙзЖаЮЦПФкЦјЬхбЙЧПМѕаЁЃЌДѓЦјбЙНЋвКЬхбЙШызЖаЮЦПЃЈЯжЯѓКЭдвђИї2ЗжЃЉ
ЃЈ3ЃЉМз ЃЈ2ЗжЃЉ
ЃЈ4ЃЉШЁЩйСПЪдвКгкЪдЙмжаЃЌЕЮМгТШЛЏБЕЛђТШЛЏИЦШмвКЃЌШєгаАзЩЋГСЕэЩњГЩЃЌПЩжЄУїгаNa2CO3ДцдкЁЃЃЈЦфЫћКЯРэЕФД№АИОљПЩЃЉЃЈ2ЗжЃЉ


 ЛюСІПЮЪБЭЌВНСЗЯАВсЯЕСаД№АИ
ЛюСІПЮЪБЭЌВНСЗЯАВсЯЕСаД№АИ бЇвЕВтЦРвЛПЮвЛВтЯЕСаД№АИ
бЇвЕВтЦРвЛПЮвЛВтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ФГПЮЭтЛюЖЏаЁзщЮЊЬНОПCO2гыЙ§СПЕФNaOHШмвКШЗЪЕЗЂЩњСЫЛЏбЇЗДгІЃЌМзЁЂввЁЂБћШ§ЮЛЭЌбЇЩшМЦСЫЯТСаШ§жжЪЕбщзАжУЃЌЧыЛиД№ЯТСаЮЪЬтЃК

(1)МззАжУжаCO2гыЙ§СПЕФNaOHЗДгІЕФРызгЗНГЬЪН ЁЃ
(2)БћзАжУЪЕбщЯжЯѓЪЧ ЃЌНтЪЭВњЩњИУЪЕбщЯжЯѓЕФдвђ
ЁЃ
(3)МзЁЂввЁЂБћЭЌбЇЩшМЦЕФШ§ИіЗНАИжаЃЌгавЛИіЗНАИдкЪЕМЪВйзїжаАВШЋадДцдкЮЪЬтЃЌИУЗНАИЪЧ (ЬюМзЁЂввЛђБћ)ЃЌдвђЪЧ
ЁЃ
(4)ЧыЩшМЦвЛИіЪЕбщМьбщЩњГЩЕФВњЮяNa2CO3жаЕФвѕРызгЁЃ(МђЪіВйзїВНжшЁЂЫљгУЪдМСЁЂЪЕбщЯжЯѓКЭНсТлЁЃ)
(5)ЪЕбщЪвжаШєашгУ44.8 L(БъзМзДЬЌ)CO2 ЃЌЯжгУКЌCaCO3 90%ЪЏЛвЪЏгызуСПЕФбЮЫсЗДгІЃЌжСЩйашвЊетжжЪЏЛвЪЏ g
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2010ФъЫФДЈЪЁГЩЖМЦпжаИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэ ЬтаЭЃКЪЕбщЬт
ФГПЮЭтЛюЖЏаЁзщЮЊЬНОПCO2гыЙ§СПЕФNaOHШмвКШЗЪЕЗЂЩњСЫЛЏбЇЗДгІЃЌМзЁЂввЁЂБћШ§ЮЛЭЌбЇЩшМЦСЫЯТСаШ§жжЪЕбщзАжУЃЌЧыЛиД№ЯТСаЮЪЬтЃК
(1)МззАжУжаCO2гыЙ§СПЕФNaOHЗДгІЕФРызгЗНГЬЪН ЁЃ
(2)БћзАжУЪЕбщЯжЯѓЪЧ ЃЌНтЪЭВњЩњИУЪЕбщЯжЯѓЕФдвђ
ЁЃ
(3)МзЁЂввЁЂБћЭЌбЇЩшМЦЕФШ§ИіЗНАИжаЃЌгавЛИіЗНАИдкЪЕМЪВйзїжаАВШЋадДцдкЮЪЬтЃЌИУЗНАИЪЧ (ЬюМзЁЂввЛђБћ)ЃЌдвђЪЧ
ЁЃ
(4)ЧыЩшМЦвЛИіЪЕбщМьбщЩњГЩЕФВњЮяNa2CO3жаЕФвѕРызгЁЃ(МђЪіВйзїВНжшЁЂЫљгУЪдМСЁЂЪЕбщЯжЯѓКЭНсТлЁЃ) 
(5)ЪЕбщЪвжаШєашгУ44.8 L(БъзМзДЬЌ)CO2 ЃЌЯжгУКЌCaCO3 90%ЪЏЛвЪЏгызуСПЕФбЮЫсЗДгІЃЌжСЩйашвЊетжжЪЏЛвЪЏ g
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2010ФъЫФДЈЪЁИпвЛЩЯбЇЦкЦкжаПМЪдЛЏбЇЪдОэ ЬтаЭЃКЪЕбщЬт
ФГПЮЭтЛюЖЏаЁзщЮЊЬНОПCO2гыЙ§СПЕФNaOHШмвКШЗЪЕЗЂЩњСЫЛЏбЇЗДгІЃЌМзЁЂввЁЂБћШ§ЮЛЭЌбЇЩшМЦСЫЯТСаШ§жжЪЕбщзАжУЃЌЧыЛиД№ЯТСаЮЪЬтЃК

(1)МззАжУжаCO2гыЙ§СПЕФNaOHЗДгІЕФРызгЗНГЬЪН ЁЃ
(2)БћзАжУЪЕбщЯжЯѓЪЧ ЃЌНтЪЭВњЩњИУЪЕбщЯжЯѓЕФдвђ
ЁЃ
(3)МзЁЂввЁЂБћЭЌбЇЩшМЦЕФШ§ИіЗНАИжаЃЌгавЛИіЗНАИдкЪЕМЪВйзїжаАВШЋадДцдкЮЪЬтЃЌИУЗНАИЪЧ (ЬюМзЁЂввЛђБћ)ЃЌдвђЪЧ
ЁЃ
(4)ЧыЩшМЦвЛИіЪЕбщМьбщЩњГЩЕФВњЮяNa2CO3жаЕФвѕРызгЁЃ(МђЪіВйзїВНжшЁЂЫљгУЪдМСЁЂЪЕбщЯжЯѓКЭНсТлЁЃ)
(5)ЪЕбщЪвжаШєашгУ44.8 L(БъзМзДЬЌ)CO2 ЃЌЯжгУКЌCaCO3 90%ЪЏЛвЪЏгызуСПЕФбЮЫсЗДгІЃЌжСЩйашвЊетжжЪЏЛвЪЏ g
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com