
| ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | �� |
���� ��Ԫ�������ڱ���λ�ÿ�֪���٢ڢۢܢݢޢߢ���ֱ�ΪC��N��O��F��Na��Al��Si��S��Cl��Ar��
��1�����ڱ������½ǵĽ�������ǿ��SiΪ�����İ뵼����ϣ�NԪ�ص���̬�⻯����������������ˮ�����ֱ�ӻ���������Σ�
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С��
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ���
��4���۵���ͼ���̬�⻯�����ΪH2O�������ͼ���̬�⻯��ΪH2S��H2O����֮�京����е�ߣ�
��5��ͬ���壬���ϵ��·ǽ����Լ�����
��6��������Ϊ��������������ǿ�����������Na�Ľ�������ǿ�����Ӧ��NaOH����������������ˮ���ﷴӦ�����κ�ˮ��
��� �⣺��Ԫ�������ڱ���λ�ÿ�֪���٢ڢۢܢݢޢߢ���ֱ�ΪC��N��O��F��Na��Al��Si��S��Cl��Ar��
��1���ϱ������½ǵĽ���Na��������ǿ������õ�Ԫ����Ar��SiΪ�����İ뵼����ϣ�NԪ�ص���̬�⻯����������������ˮ�����ֱ�ӻ���������Σ�
�ʴ�Ϊ��Na��Ar��Si��N��
��2��������ͬ�����Ų������ӣ�ԭ������������Ӱ뾶С�������Ӱ뾶����ΪF-���ʴ�Ϊ��F-��
��3���ǽ�����Խǿ����̬�⻯��Խ�ȶ�������ȶ���ΪHCl���ʴ�Ϊ��HCl��
��4���۵���ͼ���̬�⻯�����ΪH2O��ֻ��O-H���Լ���O�����Թ¶Ե��ӣ�OΪSP3�ӻ�������ΪV�Σ������ͼ���̬�⻯��ΪH2S��H2O����֮�京�������ˮ�е�ߣ�
�ʴ�Ϊ�����Լ���V���ߣ�H2O����֮�京�����
��5��ͬ���壬���ϵ��·ǽ����Լ�������ǽ����Ԣ٣��ߣ��ʴ�Ϊ������
��6��������Ϊ��������������ǿ���ᣬ����Ļ�ѧʽΪHClO4����������Na�Ľ�������ǿ�����Ӧ��NaOH�ļ�����ǿ������������������ˮ���ﷴӦ�Ļ�ѧ����ʽΪNaOH+HClO4=NaClO4+H2O���ʴ�Ϊ��HClO4��NaOH��NaOH+HClO4=NaClO4+H2O��
���� ���⿼��λ�á��ṹ�����ʵĹ�ϵ��Ϊ��Ƶ���㣬����Ԫ�ص�λ�á�Ԫ�ص����ʡ�Ԫ��������Ϊ������Ĺؼ������ط�����Ӧ�������Ŀ��飬ע��Ԫ�ػ�����֪ʶ��Ӧ�ã���Ŀ�ѶȲ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
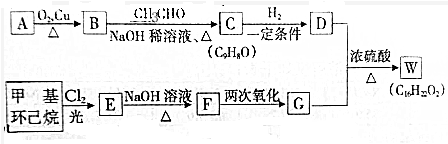
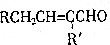 +H2O��R��R��ΪH��������
+H2O��R��R��ΪH��������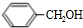 ��C�к��еĹ�����Ϊ̼̼˫����ȩ����д���ƣ���
��C�к��еĹ�����Ϊ̼̼˫����ȩ����д���ƣ���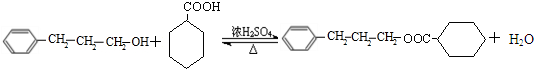 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��Ӧ�������е����������������������е������� | |
| B�� | ���տ�����0.2 mol NH3 | |
| C�� | ��Ӧ��ƽ���ʹ�ô���������ʹƽ�������ƶ� | |
| D�� | ��n��N2����n��H2��=1��3ʱ��һ���ﻯѧƽ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���Ƶ���ˮ��������ɫ���ƿ�У������������� | |
| B�� | ��ˮ����ڴ�����������ɫ�Լ�ƿ�� | |
| C�� | �ռ���Һ���ڴ���������ĥ���Լ�ƿ�� | |
| D�� | �����Ʊ�����ú���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶�/�� | 25 | t1 | t2 |
| ˮ�����ӻ����� | 1��10-14 | �� | 1��10-12 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ع���ʱ��пʧȥ���� | |
| B�� | ��������ĵ缫��ӦʽΪ��MnO2+2H2O+2e-=Mn��OH��2+2OH- | |
| C�� | ��ع���ʱ������������ͨ�����·���� | |
| D�� | ���·��ÿͨ��0.2 mol���ӣ�п�����������ϼ���6.5 g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
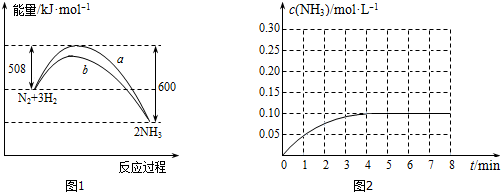
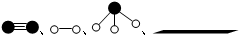 �ֱ��ʾN2��H2��NH3������������ڹ����������ϳɰ��Ĺ��̿�����ͼ��ʾ������������״̬��ߵ���B ������ĸ��ţ���
�ֱ��ʾN2��H2��NH3������������ڹ����������ϳɰ��Ĺ��̿�����ͼ��ʾ������������״̬��ߵ���B ������ĸ��ţ���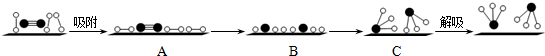
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��A��=0.5mol•L-1•mim-1 | B�� | v��B��=0.3mol•L-1•mim-1 | ||
| C�� | v��C��=0.8mol•L-1•mim-1 | D�� | v��D��=0.1mol•L-1•s-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
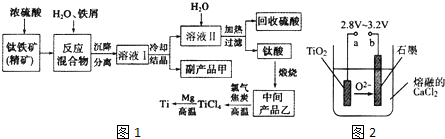
| �������� | Fe��OH��2 | TiO��OH��2 | Mg��OH��2 |
| Ksp | 8.0��10-14 | 1.0��10-29 | 1.8��10-11 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com