����⣺A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵ�ԭ��������������A��Cԭ�ӵ�L����2��δ�ɶԵ��ӣ�Ϊ̼Ԫ�غ���Ԫ�أ�ԭ������A��C������AΪCԪ�أ�CΪOԪ�أ�
B��ԭ����������A��C֮�䣬����BΪNԪ�أ�
D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��DΪMgԪ�أ�D��Eͬ���壬ԭ������D��E����EΪCaԪ�أ�
F
3+����M��3d�������Ϊ����״̬����FΪFeԪ�أ�
��AΪCԪ�أ�BΪNԪ�أ�CΪOԪ�أ�DΪMgԪ�أ�EΪCaԪ�أ�FΪFeԪ�أ�
��1��AΪCԪ�أ�BΪNԪ�أ�CΪOԪ�أ�ͬһ����Ԫ�أ�Ԫ�صĵ縺������ԭ��������������������Ե縺�Դ�С˳����C��N��O��
�ʴ�Ϊ��C��N��O��
��2��DΪMgԪ�أ�ԭ���е��ӵ��˶�״̬���������������ȣ�����þԭ�Ӻ�����12�ֲ�ͬ�˶�״̬�ĵ��ӣ���1s��2s��2p��3s���ֲ�ͬ�ܼ��ĵ��ӣ�
�ʴ�Ϊ��12��4��
��3��CΪOԪ�أ��ǽ����Խ�ǿ����Ӧ���⻯�����γ����������ˮ����֮��Ҳ�����γ����������H
2O
2�ķе��H
2S�ߣ�
CO
2����ԭ�Ӽ۲���ӶԸ�����2����������ԭ�ӵ��ӻ���ʽΪsp��ij������CO
2��Ϊ�ȵ����壬�������ڼ���Fe
3+������ΪSCN
-�������ӵĵ���ʽΪ
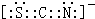
��
�ʴ�Ϊ��H
2O
2���Ӽ�����������ˮ���ӿ��γ������sp��
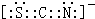
��
��4��EΪCaԪ�أ���ԭ�Ӻ�����20�����ӣ����ݹ���ԭ��֪�����������Ų�ʽΪ1s
22s
22p
63s
23p
64s
2��
�ʴ�Ϊ��1s
22s
22p
63s
23p
64s
2��
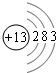
��5��������Ϊ25��Ԫ��ΪMn������Mn
2+��3d��������Ų�Ϊ����״̬���ȶ�������ʧȥ���ӣ�
�ʴ�Ϊ��Mn
2+��3d��������Ų�Ϊ����״̬���ȶ���

 ��
�� ��
��

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�
 ������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺
������Ԫ��A��B��C��D��E��F��ԭ����������������֪����A��Eͬ���壬E�ĵ�����D2��Ӧ������E2D��E2D2���ֹ��壻��F�ĵ�����D2��ȼ�յIJ����ʹƷ����Һ��ɫ��B�ĵ�����D2��ȼ�տ�����BD��BD2�������壻��CA4++DA-=CA3��+A2D�����ַ�Ӧ��������ĵ���������E+��ȣ���ش��������⣺



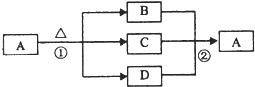
 A��B��C��D��E��F�������ʵ�ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ���δ�������
A��B��C��D��E��F�������ʵ�ת����ϵ��ͼ��ʾ����Ӧ�����Ͳ��ֲ���δ�������


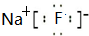
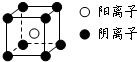
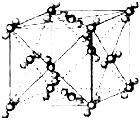 ��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ��ͼ��ʾ��E�ĵ縺���ڸ�����������أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaFΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�
��֪A��B��C��D��E��F�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E��F������B��D��Fԭ��������Ӳ��P�ܼ���������ϵĵ��Ӵ��ڰ���״̬��ͨ������£�A��һ�����������Ϊ�Ǽ��Է��ӣ��侧���ṹ��ͼ��ʾ��E�ĵ縺���ڸ�����������أ�Ga����Ԫ��B�γɵ�һ�ֻ������Ǽ���C����Ϊ�����ĵ�һ���뵼����Ϻ�GaFΪ�����ĵڶ����뵼�����֮���ڽ�10��Ѹ�ٷ�չ�����ĵ��������Ͱ뵼����ϣ�