
(10��)��ҵ
�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷�( FeSO4��7H2O )��
��1������98% 1.84 g/cm3��Ũ��������������28%��������Һ����Ũ������ˮ�������ԼΪ1�� �� ��
��2��Ϊ�ⶨij�����ڿ������̷���Ʒ��Fe2+�������ʣ�ijͬѧ�������ʵ�飺ȡһ��������Ʒ����������ϡ�����У�Ȼ�����5.00 g���۳�ַ�Ӧ���ռ���224 mL����״�������壬ʣ���������Ϊ3.88 g����÷�Ӧ�����Һ��Fe2+�����ʵ���Ϊ0.14 mol������Fe3+���������Ʒ��Fe2+���ӵ�������Ϊ �� ��
��3����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ�����������ԭ�ζ������г���������Fe2+�ı���Һ����ȡ0.4 g Cu2S��CuS�Ļ������������Һ����40 mL 0.150 mol/L KMnO4��Һ������������Ӧ���£�
8MnO4����5Cu2S��44H����10Cu2����5SO2��8Mn2����22H2O
6MnO4����5CuS��28H����5Cu2����5SO2��6Mn2����14H2O
��Ӧ�������Һ���Ͼ�SO2��ʣ���KMnO4ǡ����V mL 0.2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ����֪��MnO4����Fe2����H������Mn2����Fe3+��H2O��δ��ƽ��
��V��ȡֵ��ΧΪ �� ��
����V=35���Լ���������CuS������������
��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 -C2H5�����������ϵ��屽��ϩ��
-C2H5�����������ϵ��屽��ϩ�� -CH=CH2������ԭ����Ӧ�ǣ�
-CH=CH2������ԭ����Ӧ�ǣ� -C2H5��g��
-C2H5��g�� 
 -CH=CH2��g��+H2��g������H=+125kJ?mol-1��ij�¶��£���0.40mol
-CH=CH2��g��+H2��g������H=+125kJ?mol-1��ij�¶��£���0.40mol  -C2H5��g������2L����ܱ������з�����Ӧ���ⶨ�������ڵ����ʣ��õ��������±���
-C2H5��g������2L����ܱ������з�����Ӧ���ⶨ�������ڵ����ʣ��õ��������±���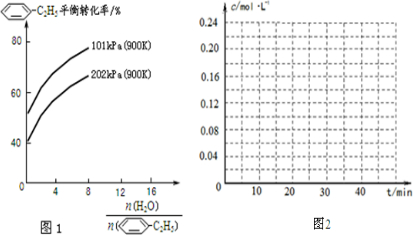
| ʱ��/min | 0 | 10 | 20 | 30 | 40 |
n�� -C2H5��/mol -C2H5��/mol |
0.40 | 0.30 | 0.26 | n2 | n3 |
n�� -CH=CH2��/mol -CH=CH2��/mol |
0.00 | 0.10 | n1 | 0.16 | 0.16 |
 -C2H5��g����ƽ��ת������ˮ��������������ϵ��ѹǿ�Ĺ�ϵ��ͼ1��ʾ����������������ʱ��ˮ����������Խ��ƽ��ת���ʽ�
-C2H5��g����ƽ��ת������ˮ��������������ϵ��ѹǿ�Ĺ�ϵ��ͼ1��ʾ����������������ʱ��ˮ����������Խ��ƽ��ת���ʽ� -CH=CH2��g����H2��g����������40minʱ�ﵽ����ͬ����ƽ��״̬������ͼ2�л�����������������
-CH=CH2��g����H2��g����������40minʱ�ﵽ����ͬ����ƽ��״̬������ͼ2�л�����������������  -C2H5��g����
-C2H5��g���� -CH=CH2��g����Ũ��c��ʱ��t�仯������
-CH=CH2��g����Ũ��c��ʱ��t�仯������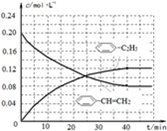

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ������2008��10��30�ձ������ڴ��ձ����ڵ���ʽ���͡���ĩ���м����˼ױ��������������Իش��������⣺
������2008��10��30�ձ������ڴ��ձ����ڵ���ʽ���͡���ĩ���м����˼ױ��������������Իش��������⣺| ���� | ��Ũ���� | ��ʯ���� ����ʯ�� |
��P2O5 | ��Ũ���� ʯ���� |
��P2O5 ʯ���� |
| KMnO4/H+��ɫ��� | ��ɫ | ��������ɫ | ��ɫ | ��ɫ | ��ɫ |
| ���Ȳ��������ʱ�� | 45s | 50s | 35s | 47s | 38s |
| ��Ӧ�¶� | 170�� | 180�� | 82�� | 170�� | 87�� |
| ���������� | �� | ���� | �� | �϶� | �϶� |
| ��ӦҺ̿����� | ���� | ��̿�� | ��̿�� | ���� | ��̿�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��
(1)��O〜30Сʱ�ڣ�CH4��ƽ������������
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g)CO(g)+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3)�÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ�����ֱ�Ϊ
��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g) CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
��2700C��ʵ��������Ӧ��������_________(����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________(����ĸ����
A. 1 〜1.5 B. 2. 5〜3 C. 3. 5〜4. 5
(5)ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ����У�Ϻ��У�����������ѧ�Ծ��������棩 ���ͣ������
�����8�֣�
ˮú�����Ƽ״��������̿�ͼ����

(ע:��ȥˮ�������ˮú����55��59%��H2��15��18%��CO��11��13%��CO2��������H2S��CH4����ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳ�)
��1����ˮú������Ҫ��ѧ��Ӧ����ʽΪ��C��s��+H2O��g�� CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ
��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ
��
�����������̼��ƽ��ת���ʵĴ�ʩ�� ��
A������C��s�� B������H2O��g�� C�������¶� D������ѹǿ
��2����CH4ת����CO����ҵ�ϳ����ô�ת���������䷴Ӧԭ��Ϊ��
CH4
(g)+3/2O2 (g) CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
CO (g)+2H2O (g) +519KJ����ҵ��Ҫѡ����ʵĴ������ֱ��X��Y��Z���ִ�����������ʵ�飨����������ͬ��
�� X��T1��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
�� Y��T2��ʱ��Ч����ߣ���ʹ����Ӧ���ʼӿ�Լ3��105����
�� Z��T3��ʱ��Ч����ߣ���ʹ�淴Ӧ���ʼӿ�Լ1��106����
��֪��T1��T2��T3������������Ϣ������Ϊ��������Ӧ��ѡ������˴����� ���X����Y����Z������ѡ��������� ��
��3���ϳ�����ѹ�����º����10m3�״��ϳ������ڴ��������£����м״��ϳɣ���Ҫ��Ӧ�ǣ�2H2(g) + CO(g)  CH3OH(g)+181.6kJ��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OH(g)+181.6kJ��T4���´˷�Ӧ��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
|
���� |
H2 |
CO |
CH3OH |
|
Ũ��/��mol��L��1�� |
0.2 |
0.1 |
0.4 |
�� �Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��v�� v�� ���>������<������)��
�� ������ͬ�����CO��H2����T5�淴Ӧ��10 min��ﵽƽ�⣬��ʱc(H2)��0.4 mol��L��1��c(CO)��0.7 mol��L��1�����ʱ���ڷ�Ӧ����v(CH3OH) �� mol��(L��min)��1��
��4�����������У��ϳ���Ҫ����ѭ������Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ֣���и�����һ������Ԥ�⻯ѧ�Ծ��������棩 ���ͣ������
(10�֣����ù��ܺ�������ɽ�CO2��H2O(g)ת��ΪCH4��O2�����������ʱ���ڲ�ͬ������I ,II��III)�����£�CH4�IJ��������ʱ��ı仯����ͼ��ʾ��

(1)
��O〜30Сʱ�ڣ�CH4��ƽ���������� ��
�� �ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
�ɴ�С��˳��Ϊ_________����Ӧ��ʼ���15Сʱ�ڣ��ڵ�_________�ִ����������£��ռ���CH4��ࡣ
(2) ������CH4��H2O(g)ͨ��۽�̫���ܷ�Ӧ����������Ӧ
CH4(g)+H2O(g) CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
CO(g)
+3H2(g) ��H=+206kJ��mol-1���������ʵ�����CH4��H2O(g)����1L�����ܱ�������ij�¶��·�Ӧ�ﵽƽ�⣬��ʱ���CO�����ʵ���ΪO.10 mol,CH4��ƽ��ת����Ϊ91 %������¶��¸÷�Ӧ��ƽ�ⳣ��Ϊ_________ (������ȡ��������
(3)
�÷�Ӧ������CO��H2�������ϳɿ�������Դ�״�����֪CO(g)��CH3OH�ŵ�ȼ���� �ֱ�Ϊ
�ֱ�Ϊ ��
�� ����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
����CH3OH(l)����ȫȼ������CO(g)��H2O(l)���Ȼ�ѧ����ʽΪ_________��
(4)��ҵ�ϳ����÷�ӦCO(g)+2H2(g)
 CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
CH3OH (g), ��H<0�ϳɼ״�����230��C〜270��C��Ϊ������Ϊ�о��ϳ�������ʵ���ʼ��ɱ�n(H2)��n(C0),�ֱ���230��C��2500C��2700C����ʵ�飬�����ͼ��
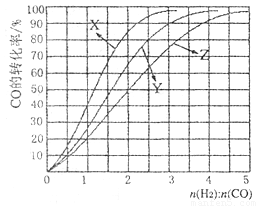
��2700C��ʵ��������Ӧ��������_________ (����ĸ����
��2300Cʱ����ҵ����������õĺϳ������n(H2):n(CO)�ı�ֵ��Χ��_________ (����ĸ����
A. 1 〜1.5 B. 2. 5〜3 C. 3. 5〜4. 5
(5) ijͬѧ��ʯīΪ�缫����KOH��ҺΪ�������Ƽ״�ȼ�ϵ�أ��为���ĵ缫��ӦʽΪ_________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com