
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Cu��OH��2 | Al��OH��3 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 5.4 | 4.0 | 1.1 | 5.8 |
| ������ȫpH | 6.7 | 5.2 | 3.2 | 8.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��ɽ��ʡΫ�������и���4���¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
��1�����µ�⼼���ܸ�Чʵ��CO2(g) + H2O(g) =CO(g) + H2(g) +O2(g)? ������ԭ��ʾ��ͼ���£�

���缫b����??????? ������������������ԭ������Ӧ��
��CO2�ڵ缫a�ŵ�ķ�Ӧʽ��?????????????????????????????? ��
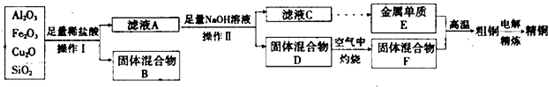
��2����ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ�������������

��֪�� Cu2O + 2H+? = Cu + Cu2+ + H2O
������ | Cu(OH)2 | Al(OH)3 | Fe(OH)3 | Fe(OH)2 |
��ʼ����pH | 5.4 | 4.0 | 1.1 | 5.8 |
������ȫpH | 6.7 | 5.2 | 3.2 | 8.8 |
����������A�еijɷ���???????????? ��
����Ӧ����ɺ���Ԫ�صĴ�����ʽΪ??????????? ���������ӷ��ţ�
��д�����ɸ����ӵ����ӷ���ʽ??????????????????????????????????????? ��
��x����ֵ��Χ��3.2��pH��4.0��y��Ӧ����ֵ��Χ��????????????? ��
�����й���NaClO��pH��˵����ȷ����???????? ������ţ���
a������NaClO��ʹ��Һ��pH����
b��NaClO�ܵ���pH����Ҫԭ�������ڷ�����ӦClO��+ H+ HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
HClO��ClO������H+���Ӷ��ﵽ����pH��Ŀ��
c��NaClO�ܵ���pH����Ҫԭ��������NaClOˮ��ClO��+ H2O HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
HClO+OH����OH������H+ ���Ӷ��ﵽ����pH��Ŀ��
��ʵ����������������Ϊ20.0%��CuSO4��Һ�����Ƹ���Һ�����CuSO4��5H2O��H2O������֮��Ϊ????????? ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£���������E������ҺC��ȡ����
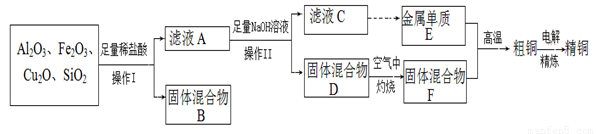
��֪��Cu2O + 2H+ = Cu + Cu2+ + H2O��
��1����ҺA����Ԫ�صĿ��ܴ�����ʽΪ_______�������ӷ��ţ�����֮��ص����ӷ���ʽΪ_____________������ҺA�д���Fe3+����������ӵ��Լ�Ϊ________�����Լ����ƣ���
��2��д��E��F��Ӧ����ͭ�Ļ�ѧ����ʽΪ_____________________________��
��3�����õ�ⷨ���д�ͭ����ʱ������������ȷ����_________������ţ���
a����������ͭ��Һ�����Һ��SO2- 4�������ƶ�
b����ͭ�ӵ�Դ������������ԭ��Ӧ
c����ͭ��������������Һ��Cu2+Ũ�ȼ�С
d������ͭ����6.4 gʱ��ת��0.2NA������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com