
(4��)��ѧ�ϳ��õ���ȱ�ʾ����ʵ����ǿ���������ѵ���ĵ���ʷ�����/��Һ��ԭ�е���ʵ��ܷ�������100%��
|
����(��)����� |
����Ȧ� |
|
A.������Һ(��һ����ȫ����)�� HSO |
10% |
|
B.����������Һ��HSO |
29% |
|
CH3COOH |
1.33% |
|
D.���HCl��H����Cl�� |
100% |
(1)25��ʱ��0.1 mol��L��1����������Һ�У�c(H��)�Ӵ�С��˳����_____(�����)��
(2)25��ʱ��0.1 mol��L��1������Һ��HSO�ĵ����С����ͬ�¶���0.1 mol��L��1����������Һ��HSO�ĵ���ȣ���ԭ����__________________��
 ��ǰ����ϵ�д�
��ǰ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(��10��)��ѧ�ϳ���ȼ�շ�ȷ���л������ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɣ���ͼ����װ������ȼ�շ�ȷ���л������ʽ���õ�װ�ã�
�ش��������⣺
��1��������������������������ѡװ�ø����ܵ�������ţ���д�ӿڴ��ţ��ǣ�
________________________________________��
��2��Cװ����ŨH2SO4��������________________________________��
��3��Dװ����MnO2��������__________________________________��
��4��ȼ�չ���CuO��������___________________________________��
��5����ȷ��ȡ 0.90g��Ʒ��ֻ�� C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�
A����������1.32 g��B����������0.54 g������л�������Ԫ�ص�����Ϊ___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ��̨һ�и߶��������¿���ѧ�Ծ����������� ���ͣ�ʵ����
(12��)��ѧ�ϳ���ȼ�շ�ȷ���л�����ɣ����ַ������ڵ�¯����ʱ�ô���������������Ʒ�����ݲ��������ȷ���л������ɣ�ͼ������װ������ȼ�շ�ȷ���л������ʽ���õ�װ�á�����������⣺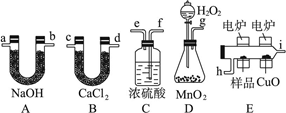
(1)������������������������ѡ���װ�ø����ܵ�����˳����________________��
(2)Cװ����Ũ�����������____________��(3)Dװ����MnO2��������__________________��
(4)ȼ�չ���CuO��������__________________��
(5)��ȷ��ȡ0.9 g��Ʒ(ֻ��C��H��O����Ԫ���е����ֻ�����)�����ȼ�պ�A����������1.32 g��B����������0.54 g������л����ʵ��ʽΪ__________________________��
(6)�����л�������ˮ�������ʽ����ԭ��Ϊ4������ṹ��ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(4��)��ѧ�ϳ��õ���ȱ�ʾ����ʵ����ǿ���������ѵ���ĵ���ʷ�����/��Һ��ԭ�е���ʵ��ܷ�������100%��
| ����(��)����� | ����Ȧ� |
| A.������Һ(��һ����ȫ����)�� HSO | 10% |
| B.����������Һ��HSO | 29% |
| CH3COOH | 1.33% |
| D.���HCl��H����Cl�� | 100% |
(1)25��ʱ��0.1 mol��L��1����������Һ�У�c(H��)�Ӵ�С��˳����_____(�����)��
(2)25��ʱ��0.1 mol��L��1������Һ��HSO�ĵ����С����ͬ�¶���0.1 mol��L��1����������Һ��HSO�ĵ���ȣ���ԭ����__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���½�ũ��ʦ����ѧ�߶���ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
(4��)��ѧ�ϳ��õ���ȱ�ʾ����ʵ����ǿ���������ѵ���ĵ���ʷ�����/��Һ��ԭ�е���ʵ��ܷ�������100%��
| ����(��)����� | ����Ȧ� |
| A.������Һ(��һ����ȫ����)�� HSO  H����SO H����SO | 10% |
B.����������Һ��HSO H����SO H����SO | 29% |
CH3 COOH COOH CH3COO����H�� CH3COO����H�� | 1.33% |
| D.���HCl��H����Cl�� | 100% |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com