
ij��ɫ��ҺX����Na+ ��Ag+ ��Ba2+ ��Al3+ ��[Al(OH)4]-- �� MnO4����CO32-- ��SO42���е�������������ϣ�ȡ��Һ���������������飺���ѧ���
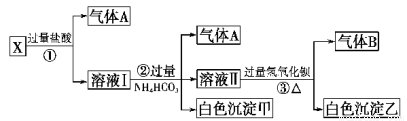
��1������A�ijɷ��ǣ�_________________������B�ijɷ���_____________
��2)X��Һ��һ�����ڵ������ǣ�____________________________
��3)д������ٷ�����Ӧ���������ӷ�Ӧ����ʽ��_________________________
��4��д��������γɰ�ɫ���������ӷ���ʽ��______________________
��5)д����ɫ�����ҵĿ�����ɣ�____________________________
��1��CO2 NH3 ��2��CO32- �� AlO2- �� Na+
��3��CO32-+2H+=H2O+CO2�� [Al(OH)4]-+4H+=Al3++4H2O
��4��Al3++3HCO3-=Al(OH)3��+3CO2�� ����5��һ������BaCO3��������BaSO4
��������
�����������1����ɫ��Һ�в����ܺ�����ɫ��MnO4������X�м���HCl,��������A��CO2����˵��ԭ������Һ�к���CO32--������CO32����Ba2+��Ag+�ᷢ�����ӷ�Ӧ�����ܵ��빲�棬��û�в���������˵������Ag+��Ba2+��CO32����Al3+�ᷢ��˫ˮ�ⷴӦҺ���ܴ������棬���Ҳ������Al3+������ҺI�м��������NH4HCO3��Һ������������������а�ɫ����������˵��������˫ˮ�ⷴӦ����ԭ��Һ�к���[Al(OH)4]-����AlO2-���������ij�����Al(OH)3��������CO2 ��������Һ�м��������Ba(OH)2��Һ����������B�ǰ��������ڢ��м����˹�����NH4HCO3��Һ������ɫ������һ������BaCO3����ԭ��Һ�к���SO42����������л��Ậ��BaSO4��������Һ�ʵ����ԣ����е�CO32- �� AlO2-���������ӣ���������Ag+ ��Ba2+ ��Al3+ �������ڣ�����һ��������Na+;��1������A�ijɷ���CO2������B�ijɷ���NH3����2)��������������֪��X��Һ��һ�����ڵ������ǣ�CO32-�� AlO2- ��Na+��һ��������������Ag+ ��Ba2+ ��Al3+ ��MnO4�������ܺ��е�������SO42������3)����ٷ�����Ӧ���������ӷ�Ӧ����ʽ��CO32-+2H+=H2O+CO2����[Al(OH)4]-+4H+=Al3++4H2O����4��д��������γɰ�ɫ���������ӷ���ʽ��Al3++3HCO3-=Al(OH)3��+3CO2������5)��ɫ��������һ������BaCO3��������BaSO4��
���㣺������Һ�ɷֵ�ȷ�������ӹ��桢�ε�ˮ�⡢�����ȷ�������ӷ���ʽ����д��֪ʶ��


 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����йذ���٤������˵����ȷ����
A����0.2mol H2SO4��Ũ����������п��Ӧ����������ķ�����С��0.1NA
B������£�22.4L���Ȼ�̼��������������NA
C��0.1mol/L ��AgNO3��Һ�У������������������ĿΪ0.1NA
D��0.2mol/L�� H2SO4��������Һ��0.1mol/L�� H2SO4��������Һ�����������ҺŨ�ȴ���0.15mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014������ʡ˫Ѽɽ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����ṹ�в�����C-C������C=C˫���Ľ���ṹ��������Ϊ֤�ݵ���( )
�ٱ�����ʹ��ˮ��Ӧ��ɫ
�ڱ�����ʹ���Ը��������Һ��ɫ
�۱���һ�������¼��ܷ���ȡ����Ӧ�����ܷ����ӳɷ�Ӧ
�ܾ��ⶨ���ڶ��ױ�ֻ��һ�ֽṹ
�ݱ�����������
A���٢ڢܢݡ�����B���٢ۢܢݡ���C���٢ڢۢܡ�����D���ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����������һ�С���һ�й��ʺ���У���������¿���ѧ�Ծ��������棩 ���ͣ������
(15��)�������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�
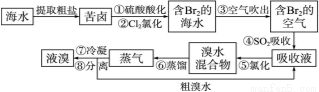
��1���������ڱ���λ�ڵ�______���ڵ�________�塣
��2����������������ữ�����Cl2�������ʣ�������__________________________��
��3�������������SO2�Ļ�ԭ�ԣ���Ӧ�����ӷ���ʽΪ________________________��
��4���������������У��¶�Ӧ������80��90 �档�¶ȹ�����Ͷ������������������ԭ��____________________________________________________��
��5���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����������������____________��
��6������١���֮��δֱ���á���Br2�ĺ�ˮ����������õ�Һ�壬���Ǿ�������������������SO2���ա������Ȼ���������������������������_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����������һ�С���һ�й��ʺ���У���������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
���й����л����˵������ȷ���ǣ� ��
A����֬���ڸ߷��ӻ�����
B�������̼ԭ�ӵ��л�������������γ��ĸ�̼̼����
C������ʽΪC8H6O2�ķ������л�������в��������Ȼ�
D�����͡�ú�ͺ�ֲ���Ͷ���̼�⻯����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����������һ�С���һ�й��ʺ���У���������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��pH=1��ϡ�����������롪������BaCl2����Һ�У�ǡ��ʹBa2+������ȫ����ʱ��Һ�����Ϊ100 mL(���ʱ��Һ����ı仯���Բ���)���һ����Һ��pH=2����ԭBaCl2��Һ��Cl-��Ũ��ԼΪ
A��0.011 mol/L B��0.22 mol/L C��0.022 mol/L D��0.11 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����������һ�С���һ�й��ʺ���У���������¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�������ӷ���ʽ��д��ȷ����
�٣�����������ʵ���Ũ�ȵ���������ϡ��Һ��̼�����ϡ��Һ���
Ba2+ + 2OH �C + NH4+ + HCO3 �C = BaCO3��+ NH3��H2O + H2O
�ڣ��Ȼ�����Һ�м�������İ�ˮ Al3+ + 4NH3��H2O = 4NH4+ + AlO2 �C + 2H2O
�ۣ�����������Һ��ͨ������Ķ������� Ca2+ + 2ClO �C + SO2 + H2O = CaSO3��+ 2HClO
�ܣ���֪��Ũ�ȵ�̼���ơ��������ơ�̼������PH��С�������������Һͨ����������̼
ClO- +CO2 +H2O = HClO + CO32-
�ݣ��������ƹ�����ˮ��Ӧ��2O22?+2H2O��4OH?+O2��
�ޣ���ˮ��ͨ�������������2NH3?H2O + SO2 = 2NH4+ +SO32? +H2O
�ߣ������ʯ��ˮ�м��˹�����NaHCO3��Һ��Ca2++OH?+HCO3?=CaCO3��+H2O
�࣮��2mol/LAlCl3��Һ��7mol/LNaOH��Һ�������ϣ�
2Al3����7OH?��Al(OH)3����AlO2?��2H2O
�ᡢ��Ba(OH)2��Һ�еμ�NaHSO4��Һ��ǡ��Ϊ���ԣ�Ba2+ + 2OH- + 2H+ + SO42- = BaSO4��+ 2H2O
A���٢ۢޢ� B���ڢܢݢ� C���ۢܢߢ� D���٢��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ��һ�ڶ�ѧ����ĩ��ѧ�Ծ��������棩 ���ͣ������
��8�֣�������з���ʽ��Ҫע����Ӧ��������
�ټ�����������Ӧ��һ�ȼ��飺________________________ ����ϩʹ��ˮ��ɫ��_______________
�۱���Һ���ϼ����� ��__________________���Ʊ�����������_________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014����ʡ�Ӱ�����ѧ����ĩ����һ��ѧ�Ծ��������棩 ���ͣ������
��8�֣��������ʵ�����A��B�����2 L���ܱ������У��������·�Ӧ�� 3A(g)��B(g) xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c (A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
xC(g)��2D(g)����5 min���D��Ũ��Ϊ0.5 mol/L��c (A)��c(B)��3��5��C��ƽ����Ӧ����Ϊ0.1 mol/(L��min)����
��1����ʱA��Ũ��c(A)��________mol/L����Ӧ��ʼǰ�����е�A��B�����ʵ�����n(A)�� n(B)��________mol��
��2��B��ƽ����Ӧ����v(B)��________mol/(L��min)��
��3��x��ֵΪ________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com